So far, more than 10 types of antibody-drug conjugates (ADC) have been approved by the U.S. Food and Drug Administration (FDA) and have contributed to the medical field. Interestingly, they are all manufactured using chemical conjugation techniques. This indicates that chemical conjugation is the primary method for ADC manufacturing. Compared to other conjugation techniques such as genetic engineering or enzymatic conjugation, chemical conjugation has the advantage of relatively simple chemistry, manufacturing, and control (CMC), which encourages pharmaceutical companies to choose this technique for ADC project production. To better understand this advantage, this article outlines the mature all-chemical coupling methods that have been applied in the scale-up phase of ADC production.
1. Random Conjugation Previously, the most popular method for manufacturing ADC was chemical random conjugation. This method utilizes random modifications, where natural lysine on the antibody surface or cysteine on interchain disulfide bonds is reduced. Natural lysine conjugation is the traditional method of linking drug linkers to antibodies.
Active ester groups (e.g., N-hydroxysuccinimide) react with primary amines on exposed lysine residue side chains, forming stable covalent bonds. This technology has been used to produce four FDA-approved ADCs (gemtuzumab ozogamicin, trastuzumab emtansine, inotuzumab ozogamicin, and mirvetuximab soravtansine).

Utilizing the lysine residues of antibodies, the nucleophilic NH2 groups of amino acids react with the electrophilic N-hydroxysuccinimide (NHS) groups on the linker. Although the reaction is simple, the high abundance of lysine residues can lead to the formation of uneven mixtures of many ADCs distributed randomly.
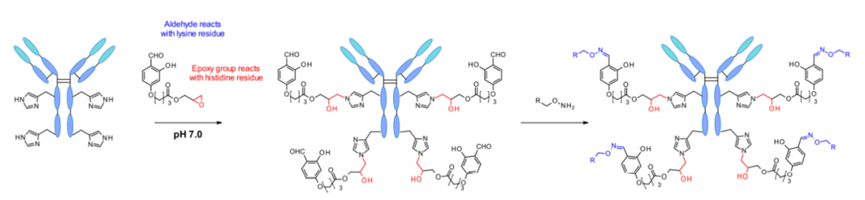
The reagent carries multiple functional groups that reversibly form imine bonds on all available lysine residues. Then, the key protein reacts with proximal histidine residues through the epoxide in the reagent. Thus, under physiological conditions, the key protein is separated from lysine, and the aldehyde is regenerated, enabling the labeling of antibodies through oxime conjugation.
In contrast, natural cysteine conjugation is referred to as the “semi-random” conjugation technique, which utilizes cysteine generated by treating partially reduced interchain disulfide bonds with controlled equivalents of reducing agents such as tris(2-carboxyethyl)phosphine. This controlled reduction, followed by the conjugation strategy of thiol-maleimide coupling, has been applied to produce five marketed ADCs (brentuximab vedotin, polatuzumab vedotin, enfortumab vedotin, belantamab mafodotin, and tisotumab vedotin).

This cysteine-based method can be considered more advantageous than the lysine-based method as it simplifies CMC and allows for “partial” control over the drug-antibody ratio (DAR) and ADC heterogeneity.
To minimize issues arising from ADC heterogeneity, several site-specific conjugation techniques have been developed. Below are three promising chemical conjugation techniques for producing site-specific ADCs.
2. Site-Specific ConjugationADC In 2019, with the FDA approval of trastuzumab deruxtecan, site-specific ADCs entered the clinical arena for the first time. In manufacturing this ADC, Daiichi Sankyo overcame challenges associated with DAR of 8 for ADCs that were previously considered too hydrophobic and impractical. They successfully created a stable ADC even at a DAR of 8 by using a unique combination of payload and linker.
The conjugation technology used mimics traditional cysteine-based ADCs, as it utilizes the reduction of interchain disulfide bonds (although all disulfide bonds are cleaved). However, random ADCs contain positional isomers, making quality control, reproducibility, and analysis difficult. In contrast, DAR of 8 for ADCs has the maximum number of cysteine groups conjugated with payload, minimizing their heterogeneity.
For Daiichi Sankyo’s payload, a irinotecan analog was chosen to generate hydrophilic carboxylic acids in circulating blood. This payload is covalently linked to the antibody through an enzymatically cleavable linker: a tetrapeptide (Gly Gly Phe-Gly). This tetrapeptide linker provides promising characteristics with high contrast between plasma stability and sensitivity to specific enzymes present in cancer cells. The combination of these factors enhances the stability of high DAR ADCs.
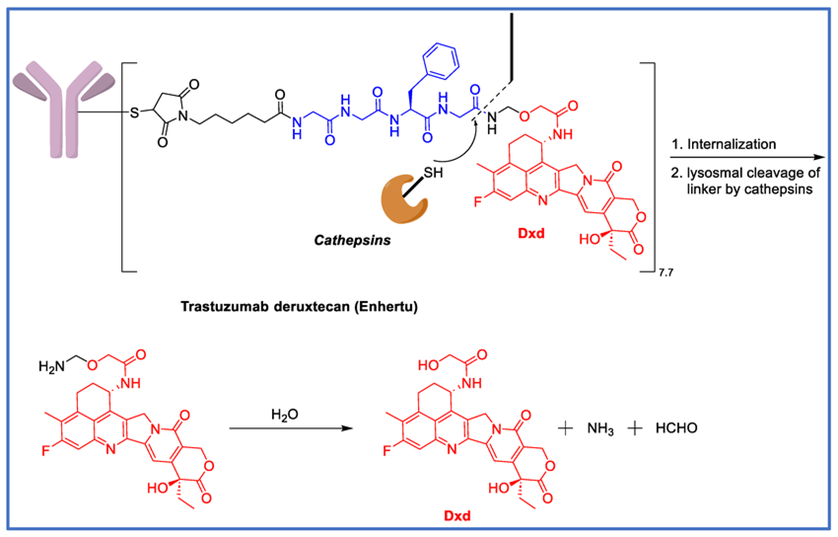
fam-trastuzumab deruxtecan-nxki or DS-8201a, DAR=8

sacituzumab govitecan
In 2014, the PolyTherics research group (now part of Abzena) reported a unique chemical conjugation method called ThioBridge®. After the reduction of interchain cysteine, this bridging technology provides selective and stable covalent bonds with a DAR of 4 for ADCs. Using this method, the overall process yield exceeds 70%. Furthermore, they established an appropriate separation strategy using preparative HPLC to obtain homogeneous DAR ADCs with higher purity.

ThioBridge linkage
Affinity labeling is a method using affinity compounds, which is a mature tool in the field of chemical biology. In 2019, the Ajinomoto Group adopted this method to produce site-specific ADCs by modifying the Fc region of antibodies using specific affinity reagents. This technology, named AJICAP, quickly gained good laboratory practices (GLP) certification for producing relevant preclinical studies.
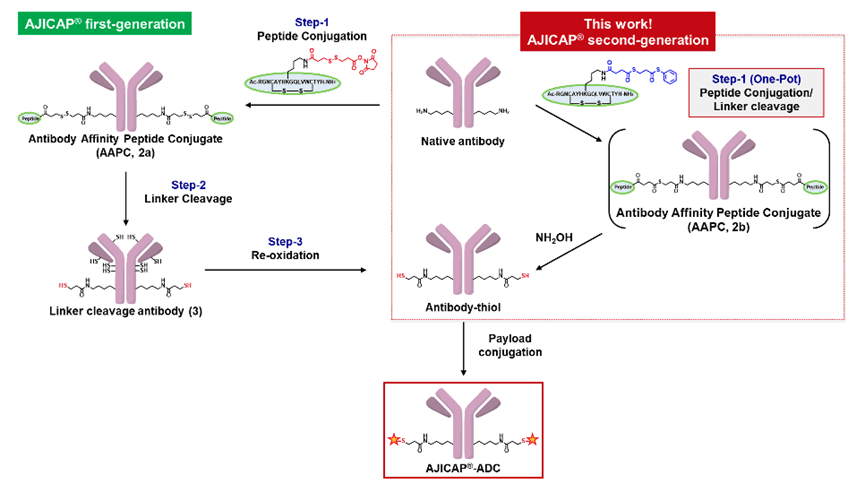
AJICAP® conjugation technology
These three chemical site-specific conjugation methods have matured significantly, and their notable characteristics are summarized in the table below (Table 1.0).
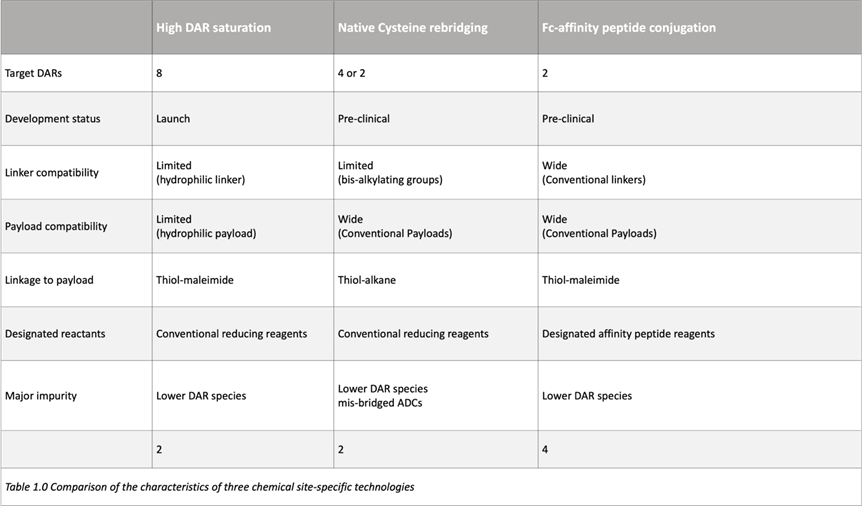
Methods with DAR 8 have significant advantages in the track record of technologies used in approved ADCs. However, the capabilities of this technology appear to be limited, especially concerning target DAR and drug linker compatibility.
For example, the main drug linker in clinical ADCs is MC-VC-PAB-MMAE, which cannot be applied to this high DAR method without redesigning the drug linker. The hydrophobicity of MC-VC-PAB-MMAE leads to significant aggregation, reducing its pharmacokinetics and safety. Furthermore, due to this conjugation chemistry, the hinge region lacks disulfide bonds, which may lead to potentially hazardous changes in the physical properties of the resulting ADCs compared to naked antibodies. Therefore, sacituzumab govitecan-hziy (Trodelvy®; Gilead Oncology, approved in 2020) has significantly lower antibody-dependent cytotoxic activity than its naked antibody. Both ADCs have been approved by the FDA, indicating that this physical change caused by the hinge disulfide bond is not an inherent issue; however, it may limit the combination of antibodies and drug linkers in high DAR ADCs.
3. Cysteine Rebridging Cysteine rebridging is an excellent method for forming stable covalent bonds while overcoming issues caused by reverse Michael reactions. This side reaction releases payload into the bloodstream, leading to adverse toxicity. However, a potential drawback of cysteine rebridging is the formation of some undesired “misbridged” ADCs during the conjugation reaction, leading to reaction-related heterogeneity. The use of novel dialkylation reagents is likely to solve this potential issue.
The Fc affinity compound method shows great promise, as it can be applied to various antibodies and drug linkers without optimizing reactions. Furthermore, this conjugation method can provide site-specific ADCs with a DAR of 2, without the need for hydrophilic linkers or dialkylation reagents. However, a potential drawback of this conjugation technique is the need for a relatively long synthetic sequence. The Ajinomoto research group indicates that recent optimizations have been made to simplify the sequence, which may resolve this issue.

Using (A) dialkylsulfide, (B) next-generation maleimide (NGM), (C) dioxopiperazine (PD) or (D) “two-in-one (2-in-1)” PD reagents to generate homogeneous ADCs via disulfide rebridging are selected examples.
4. Conclusion In conclusion, all three conjugation methods can be recommended to drug developers for specific ADC pipeline projects based on specific targets (e.g., biological properties, target DAR, etc.).
Disclaimer: The publication/reprinting of this article is solely for the purpose of disseminating information and does not imply the representation of the views of this public account or confirm the authenticity of its content. Any judgments made based on this content are at your own risk. If there is any infringement, please inform us and we will delete it!
Long press to follow this public account

