Antibody-drug conjugates (ADC) have become one of the most sought-after methods for treating cancer patients. Recently, several notable deals have taken place between small biotech companies developing ADCs and large companies looking to enhance their oncology pipelines.
The recent Kangfang AK112 incident has brought bispecific antibodies into the spotlight, with some discussing the viability of innovative drugs and others questioning the feasibility of bispecific antibodies.
The competition in the ADC field is fierce, and a key question for companies developing ADCs is how to stand out from the competition. Since most ADCs currently under development are monospecific antibodies targeting a single antigen/target, one way to differentiate from competitors is to develop ADCs that bind to two or more antigens or epitopes on the same target, known as bispecific ADCs.
When developing bispecific ADCs, at least two important questions need to be addressed. The first question is whether bispecific ADCs have superior efficacy and safety compared to monospecific ADCs used alone or in combination. The second question is whether developing bispecific ADCs is cost-effective, considering the complexity involved.
Classification of ADCs
ADCs can generally be divided into four major categories:
The first is the traditional monospecific ADC, where the ADC has high affinity for a single epitope on the cell surface antigen/target.
The second is biparatopic ADC, which binds to two different epitopes on the same antigen/target.
The third is bispecific ADC, which binds to two different cell surface antigens/targets on tumors.
The fourth category is bispecific antibody fragments, which use smaller antibody scaffolds to enhance antibody penetration into solid tumors. Bispecific ADCs may represent the next wave of clinical development.
How to Select Targets?
There are currently very few bispecific ADCs in clinical development, with about ten bispecific or biparatopic ADCs. How to choose which antigens to target with bispecific ADCs? Starting from the ADCs that have received FDA approval (BCMA, Nectin-4, CD19, CD22, CD33, CD79b, CD30, TROP2, Her2, tissue factor, and folate receptor-a), there are 11 * 11 matrices or 56 possible combinations. Focusing on tumor-expressed limited targets, such as folate receptor (ovarian cancer, fallopian tube cancer, peritoneal cancer) and Nectin-4 (bladder cancer and kidney cancer) or hematological targets, forms a 3 * 3 matrix (HER2, TROP2, and tissue factor) or six possible combinations.
The following image shows bispecific ADCs currently in clinical development. We can see JSKN-003 (HER2x HER2) and Zalontamab brengitecan (BL-B01D1) (EGFRxHER-3) in Phase III, TQB2102 (HER2x HER2) in Phase II, and others in Phase I.
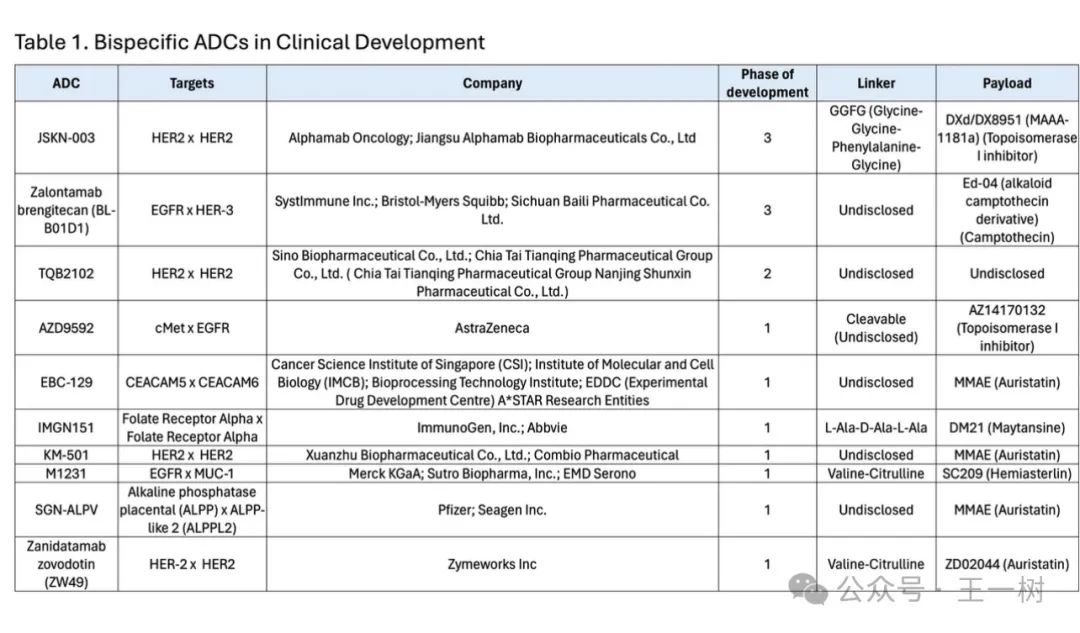
To expand the number of potential bispecific targets, it is advisable to focus only on ADC targets that have entered Phase III clinical trials, as these targets are clinically validated. Currently, about eight ADC targets have entered Phase III, providing 36 possible bispecific and biparatopic ADC target combinations.
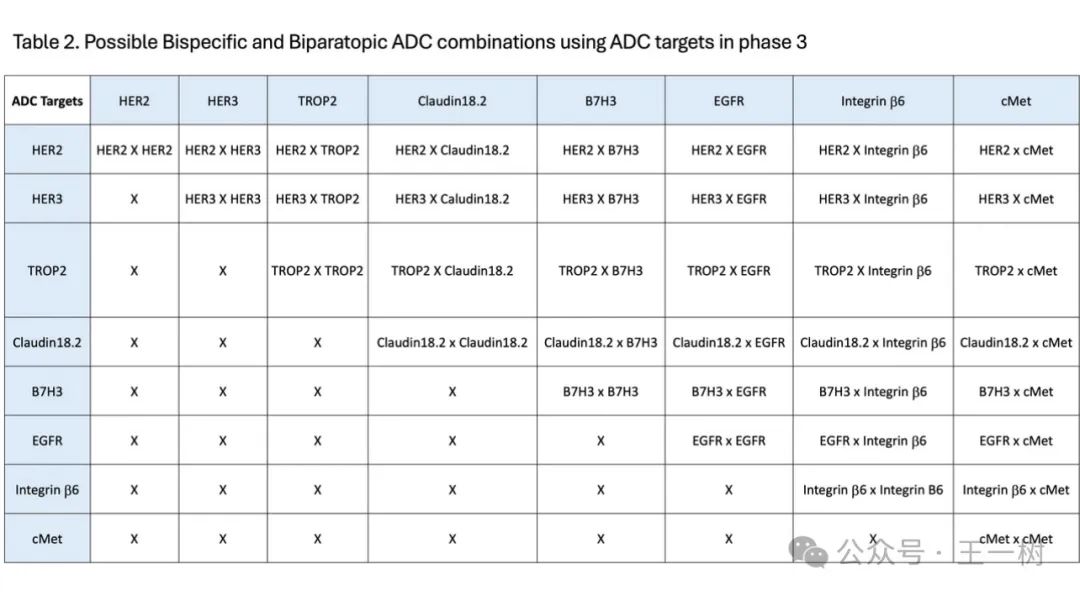
As ADC targets are incorporated into the early stages of clinical development, the number of possible target combinations will increase. There are approximately 13 ADC targets in Phase II for solid tumor indications, which will have the potential for over 100 possible combinations. Most bispecific ADCs, including some biparatopic ADCs, are still in early clinical development stages.
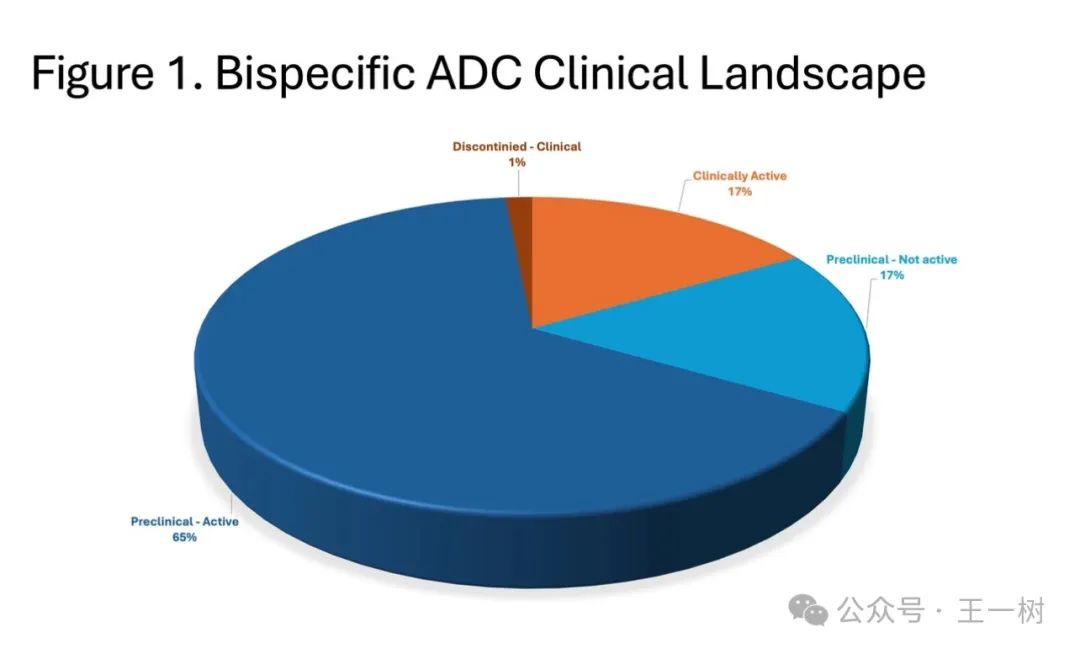
As ADC targets are included in the early stages of clinical development, the number of potential target combinations will increase. There are approximately 13 ADC targets in Phase II studies for solid tumor indications, which will have the potential for over 100 possible combinations. Most bispecific ADCs and some biparatopic ADCs are in early clinical development.
Choosing targets in early clinical development may increase the risk of failure due to a lack of clinical validation. It is worth mentioning that most tumor drug development, including ADCs, does not progress beyond Phase II into Phase III.
How to Determine Which Targets to Combine?
With many possible bispecific target combinations, how should one determine which targets to combine? The following three methods can be referenced: One method is to identify co-expressed targets in the literature for the tumor indications of interest.
Zalontamab brengitecan (BL-B01D1) is a bispecific ADC that targets both EGFR and HER3. BL-B01D1 is in Phase III in China and Phase I in the United States. EGFR is expressed in 52.3%, and HER3 is expressed in 82.7% of primary tumors in non-small cell lung cancer (NSCLC) patients. EGFR is expressed in 62.7%, and HER3 is expressed in 91.2% of brain metastases. Another method is to use single-cell transcriptome analysis and multi-group cross-validation. This method was used to identify 11 new cell surface targets for multiple myeloma. Additionally, bioinformatics techniques can be used to identify potential bispecific targets. Co-expression analysis can be used to identify potential bispecific antibody targets.
Cell Surface Antigen Binding, Internalization, and Intracellular Transport
Bispecific ADCs can induce aggregation on the cell surface, enhancing antibody internalization and lysosomal delivery. Data from Her2 biparatopic ADCs and Met bispecific ADCs report enhanced receptor aggregation and lysosomal transport. For example, MEDI4276 is a Her2 biparatopic ADC that reportedly enhances lysosomal transport and in vitro cytotoxicity compared to monospecific Her2 ADCs. The preclinical data for MEDI4276 shows great promise, but its clinical development ended at Phase II.
Different ADC targets internalize at different rates. This may affect the response of ADCs, sometimes favoring one target over another. Another consideration is the affinity of each arm for the antigen. In some cases, it may be necessary to reduce the affinity of one antigen and increase the affinity for another antigen to minimize toxicity to the target in normal tissues. EGFR is an example where, compared to EGFR ADCs with higher affinity, RN765C has lower affinity for EGFR, resulting in reduced in vitro cytotoxicity to normal keratinocytes while maintaining strong in vivo antitumor efficacy in EGFR-expressing tumor xenograft models.
Challenges in Bispecific Antibody Format and Production
The main issue in producing bispecific antibodies is ensuring the formation of bispecific antibodies with high efficiency and sufficient yield. Currently, several bispecific antibody formats are available for ADCs.
Some bispecific antibody formats require two cell lines to stably produce each arm of the antibody. Each arm of the antibody will be purified, and after purification, assembled in vitro to produce bispecific antibodies. This method requires engineering each arm of the antibody, for example, using knob-into-hole formats to ensure proper assembly of bispecific antibodies through favorable thermodynamics. Some bispecific antibody formats require extensive antibody engineering to ensure correct formation of heterodimeric antibodies. IMGN151 is a bispecific ADC targeting folate receptor a, containing VH44-VL100 cysteine mutations to prevent scFv aggregation, and has a C220S mutation in the Fc domain to reduce the formation of free cysteine. Determining the correct expression structure and expression format may be a challenge compared to stable single-cell expression.
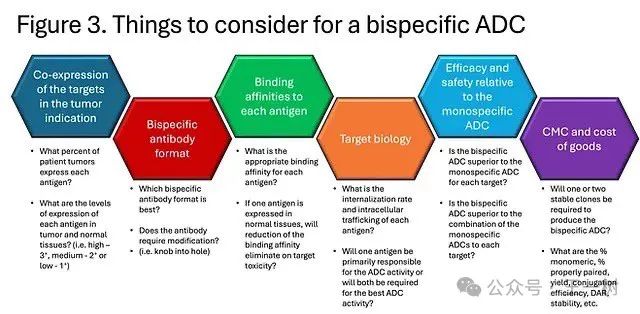
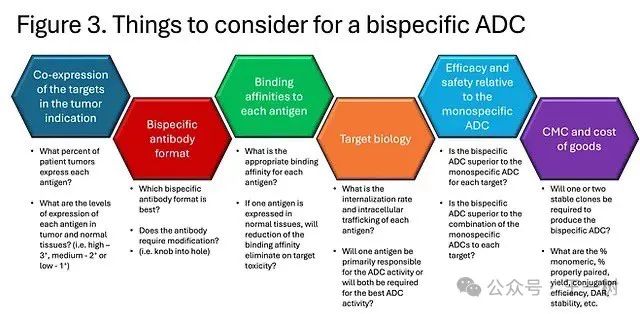
Some considerations when developing bispecific ADCs are illustrated in the following figure. It is crucial to demonstrate that bispecific ADCs can provide better efficacy and safety compared to monospecific ADCs. Comparing monospecific ADCs alone and in combination will be an important control group in preclinical studies. It is also important to show that bispecific ADCs outperform monospecific ADCs and combinations of monospecific ADCs in tumor models with heterogeneous expression of each antigen. Finally, antibodies with similar binding affinity to human and non-human primate antigens will also be important for non-GLP and GLP toxicology studies.
Conclusion
Due to the heterogeneity of ADC target expression in tumors, bispecific ADCs provide another approach to address potential issues of drug resistance. Theoretically, bispecific ADCs may also enhance tumor targeting as the expression of both antigens in tumors may be higher compared to normal tissues. Bispecific ADCs, including biparatopic ADCs, may improve ADC internalization rates and lysosomal transport, potentially making them more effective than monospecific ADCs. For monospecific ADCs, when the expression of one ADC target is low, the drug may become ineffective; thus, targeting two tumor antigens with one ADC can minimize the likelihood of bispecific ADC failure when the expression rate of one tumor antigen is low.
Currently, the development of bispecific ADCs is mostly in early clinical stages. Preclinical candidates such as JSKN-003 (Her2 biparatopic) and BL-B01D1 (EGFR x Her3) are in Phase III clinical trials in China, while they are still in early clinical development in the United States. It remains to be seen whether these bispecific ADCs will have significant advantages over monospecific ADCs.
The production challenges of bispecific ADCs need to be considered, as the antibody format, chosen linker/payload, and coupling methods can all pose significant challenges. Strict QC standards need to be implemented from the early stages of development to minimize the risk of batch manufacturing failures later in production, as this can lead to costly losses and significant delays.
Bispecific ADCs enhance targeting of tumors while reducing damage to normal tissues, theoretically providing better efficacy and safety than monospecific ADCs. The ADC field is highly competitive, and differentiating from competitors may not be easy, but bispecific ADCs may provide an important differentiator in the ADC landscape, ultimately benefiting cancer patients.



