▎Armstrong
The 2025 American Association for Cancer Research (AACR) Annual Meeting will be held from April 25-30 in Chicago, USA. Recently, the conference abstracts have been released, showcasing a significant presence of Chinese innovations. In the field of ADCs, the majority of reports come from Chinese pharmaceutical companies, with nearly 100 innovative ADCs covering various technological routes and targets, truly leading the innovation frontier of ADCs.
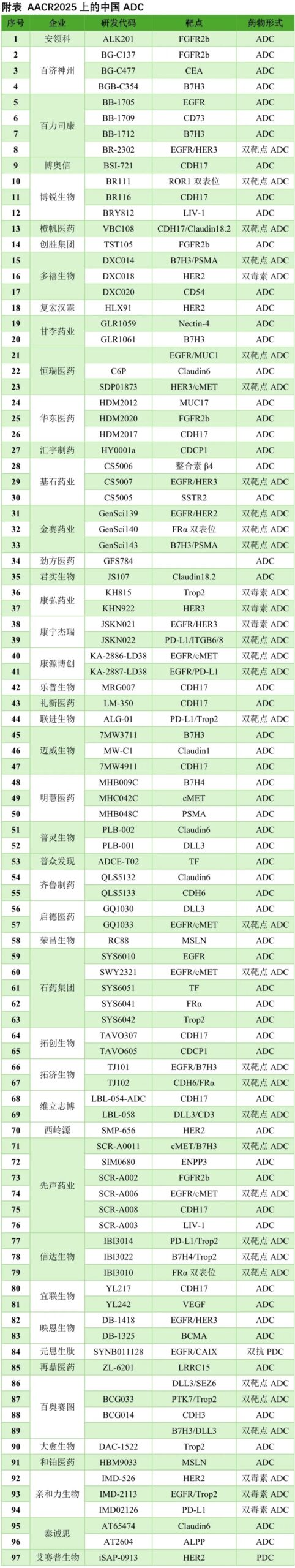
Bispecific ADCs are currently one of the hottest directions in the ADC field, with companies such as BaiLisiKang, BaiAoSaiTu, BoRui Biotech, ChengFan Pharma, DuoXi Biotech, HengRui Pharma, JiShi Pharma, JinSai Pharma, KangNingJieRui, KangYuan BoChuang, LianJin Biotech, QiDe Pharma, ShiYao Group, TuoJi Biotech, XianSheng Pharma, XinDa Biotech, YingEn Biotech, and Affinity Biotech all making strides in this area.
Bispecific toxin ADCs are also coming to the forefront, with KangHeng Pharma and Affinity Biotech developing bispecific toxin ADCs. KangHeng Pharma’s KH815 is the world’s first bispecific toxin ADC new drug to enter clinical stages. The two payloads are TOP1i and RAN POL2i, which simultaneously inhibit RNA synthesis and induce DNA double-strand breaks.

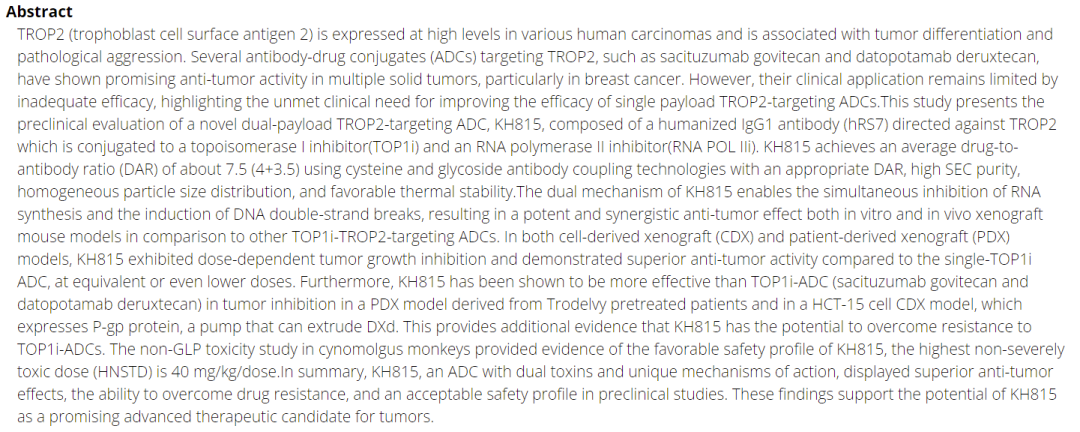
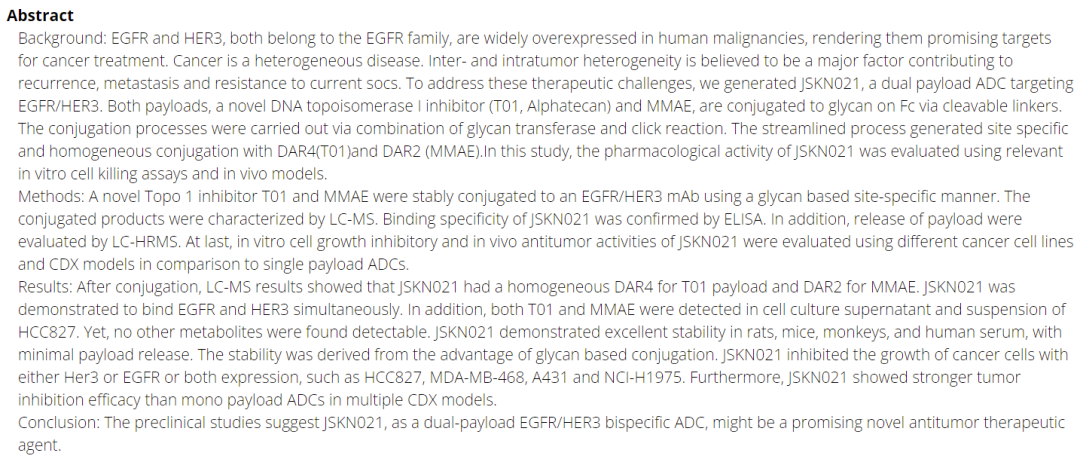
KangNingJieRui has further developed a bispecific bispecific toxin ADC, namely the EGFR/HER3 bispecific toxin ADC new drug JSKN021.

The traditional concept of ADCs is that toxins are released after antibodies bind to receptors and mediate endocytosis. DXd-ADC has demonstrated the significant role of the bystander effect, and YiLian Biotech has further developed a non-endocytosed ADC that targets free VEGF, releasing toxins through tumor microenvironment-specific enzymes, further expanding the application space of ADC drugs.


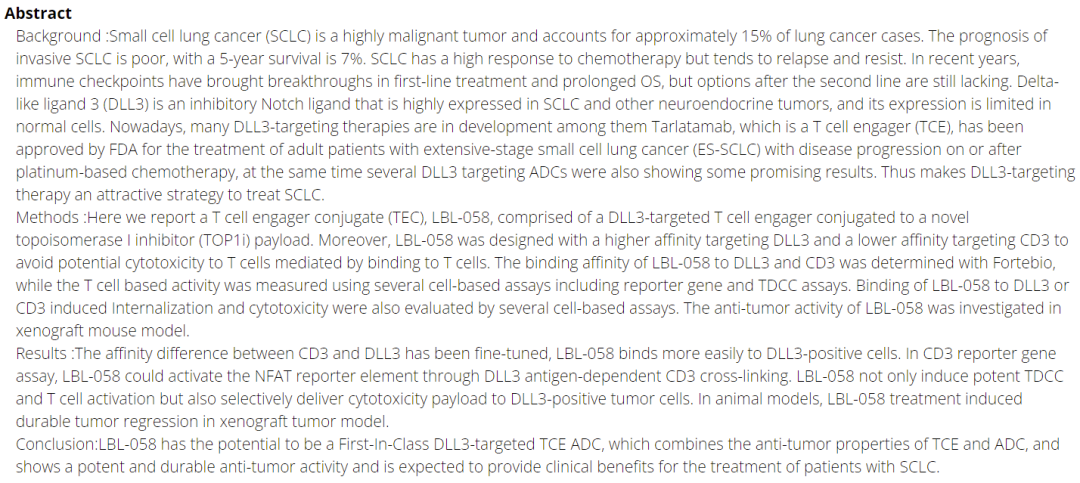
WeiliZhiBo has developed the first TCE ADC, namely the DLL3/CD3 ADC new drug LBL-058, which is formed by coupling DLL3/CD3 bispecific antibodies with TOP1i, synergizing T-cell killing and payload cytotoxic effects. Although TCE has begun to break through solid tumors at a few targets, the design of TCE-ADC undoubtedly provides a new enhanced design approach for overcoming solid tumors.

Conclusion
From this AACR meeting, it is evident that Chinese ADCs are leading the innovation frontier in terms of both quantity and differentiated design. New target ADCs, new target combination bispecific ADCs, bispecific toxin ADCs, PDCs, TCE-ADCs, non-endocytosed ADCs, etc., along with self-immune ADCs (YingEn Biotech has already entered clinical stages) not covered in the AACR meeting, are all noteworthy. In the coming years, the clinical breakthroughs and international transactions of domestic ADCs remain a focal point of the industry.

Armstrong Technology Overview Series
-
Comprehensive Overview of GPRC5D Target;
-
Comprehensive Overview of CD40 Target;;
-
Comprehensive Overview of CD47 Target;;
-
Comprehensive Overview of Complement Targeted Drug Technology;
-
Complement Drugs: An Important Direction for Ophthalmic Treatment;
-
Comprehensive Overview of Claudin 6 Target;
-
Comprehensive Overview of Claudin 18.2 Target;;
-
Target Warm and Cold, Industry Self-Awareness;
-
China’s Large Molecule New Drug R&D Landscape;
-
Criticism of “Me Too”;
-
History of Adjuvants;;
-
Legend of Insulin Over a Century;
-
CUSBEA: Forty Years of Storms;
-
China’s Anxiety in New Drug R&D
-
R&D Competition Among Chinese Biopharmaceutical Companies;
-
Competition Landscape of Chinese Bispecific Antibodies;;
-
Competition Landscape of Chinese ADCs;;
-
Comprehensive Overview of Chinese Bispecific Antibody Technology;
-
Comprehensive Overview of Chinese ADC Technology;
-
Comprehensive Overview of Ambrx Technology;
-
Comprehensive Overview of Vir Biotech Technology;
-
Comprehensive Overview of Immune-Onc Technology;
-
Comprehensive Overview of Gengxi Biotech Technology;
-
Comprehensive Overview of KangZhe Pharma Technology;
-
Comprehensive Overview of Keji Pharma Technology;
- Comprehensive Overview of KaiKa Biotech Technology;
- Comprehensive Overview of Tongyi Pharma Technology;
- Comprehensive Overview of BaiAoSaiTu Technology;
- Comprehensive Overview of Tengsheng BoYao Technology;
- Comprehensive Overview of Chuangsheng Group Technology;
- Comprehensive Overview of YongTai Biotech Technology;
- Comprehensive Overview of Chinese Antibody Technology;;
- Comprehensive Overview of Deqi Pharma Technology;
- Comprehensive Overview of Deqi Pharma Technology 2.0;
- Comprehensive Overview of HeBo Pharma Technology;;
- Comprehensive Overview of RongChang Biotech Technology;;
- Comprehensive Overview of ZaiDing Pharma Technology;;
- Comprehensive Overview of WuXi Biologics Technology;;
- Comprehensive Overview of HengRui Pharma Technology;;
- Comprehensive Overview of Haosen Pharma Technology;;
- Comprehensive Overview of ZhengDa Tianqing Technology;;
- Comprehensive Overview of GigaGene Technology;
- Comprehensive Overview of ZhenJiang Pharma Technology;
- Comprehensive Overview of ZhenJiang Pharma Technology 2.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 3.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 4.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 5.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 6.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 7.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 8.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 9.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 10.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 11.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 12.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 13.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 14.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 15.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 16.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 17.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 18.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 19.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 20.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 21.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 22.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 23.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 24.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 25.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 26.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 27.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 28.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 29.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 30.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 31.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 32.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 33.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 34.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 35.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 36.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 37.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 38.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 39.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 40.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 41.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 42.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 43.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 44.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 45.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 46.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 47.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 48.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 49.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 50.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 51.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 52.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 53.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 54.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 55.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 56.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 57.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 58.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 59.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 60.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 61.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 62.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 63.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 64.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 65.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 66.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 67.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 68.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 69.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 70.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 71.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 72.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 73.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 74.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 75.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 76.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 77.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 78.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 79.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 80.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 81.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 82.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 83.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 84.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 85.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 86.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 87.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 88.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 89.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 90.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 91.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 92.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 93.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 94.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 95.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 96.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 97.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 98.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 99.0;
- Comprehensive Overview of ZhenJiang Pharma Technology 100.0;