
Due to recent changes in the public account push mechanism, we welcome you to set Boom Health as a 🌟”Starred”🌟 account on the homepage to receive the latest trends and expert insights in the digital healthcare industry!
This article is approximately 4300 words long and will take about 10 minutes to read.
On April 10, 2025, the FDA released the “Roadmap to Reducing Animal Testing in Preclinical Safety Studies” [1], announcing plans to gradually replace animal testing requirements for monoclonal antibodies and other drugs. In the future, more effective and human-relevant experimental methods will replace animal testing, aiming to improve drug safety, accelerate evaluation processes, reduce animal testing, and lower R&D costs.
The release of the FDA’s latest policy implementation roadmap has further increased the industry’s interest in organoids, organ-on-chip technologies, and AI in drug development. In 2025, ARK Invest’s annual report “Big Ideas 2025” also specifically mentioned the value of combining AI with organoids/organ-on-chip technologies to accelerate pharmaceutical R&D. The combination of organoids/organ-on-chip and artificial intelligence can facilitate the efficient development of innovative drugs, helping pharmaceutical companies navigate the “valley of death” in R&D.
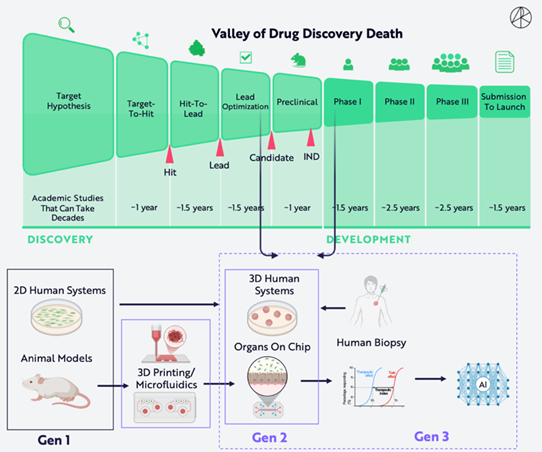
1. Reforming Drug Development Models — Concepts and Advantages of Organoids and Organ-on-Chip
The FDA’s report points out that animal models are insufficient to adequately simulate human health and disease, with over 90% of drugs that appear safe and effective in animal models ultimately failing to gain FDA approval for human use. Animal testing data is particularly inaccurate for predicting various common diseases, including cancer, Alzheimer’s disease, and inflammatory diseases. There are significant physiological differences between humans and other animal species.
The new methodologies recommended by the FDA to replace animal testing (NAMs) are mainly divided into two categories: organoids and organ-on-chip technologies, and AI predictive models. Both organoids and organ-on-chip technologies are collectively referred to by the FDA as microphysiological systems. Both are cultivated based on human cells and can effectively simulate the physiological functions of human organs and multiple organs, thus providing opportunities for mechanistic research within a “human model” system. These systems effectively eliminate species-specific mechanisms and provide valid alternatives to animal models for studying human organs. The FDA believes that microphysiological systems have predictive capabilities for human responses that are comparable to or even superior to animal testing.

A search for the term “organoid” on Pubmed shows that related articles have surged from 189 in 2014 to 4202 in 2024, making organoids a rapidly growing focus in life sciences research. The number of papers related to the term “organ-on-chip” has also increased from 174 in 2014 to 794 in 2024.
In 2013, Science named organoids as one of the top ten technologies of the year, marking the first time organoid technology was included in the annual list of top scientific achievements due to its breakthrough in overcoming the limitations of traditional 2D cultures. In 2015, it was selected as one of MIT Technology Review’s top ten breakthrough technologies. In 2017, organoids were included in Nature Methods’ annual methods, driving a paradigm shift in precision medicine research. In 2019, they were recognized as a high-value preclinical model by NEJM, establishing their clinical value as an alternative to animal testing.
Organoids refer to organ-like structures formed by the self-differentiation of adult or pluripotent stem cells through three-dimensional culture, possessing histological characteristics similar to human organs and capable of reproducing key functions, structures, and biological complexities of the organs.
Organ-on-chip refers to microfluidic chips that can co-culture cells or mini-tissues and provide a controllable microenvironment, thereby achieving key functions of organs or multiple organs in vitro.
The advantages of organoids/organ-on-chip technologies include high predictive accuracy, the ability to simulate humanized tissue functions, and a high degree of restoration of individual organ composition and function. Compared to animal models, they also offer high construction efficiency, lower costs, and the ability to maintain genetic and phenotypic stability in a high-throughput manner.
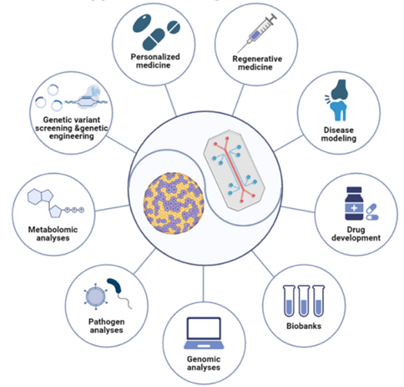
2. Increased Policy Support — Regulatory Policies Related to Organoids and Organ-on-Chip Technologies Domestically and Internationally
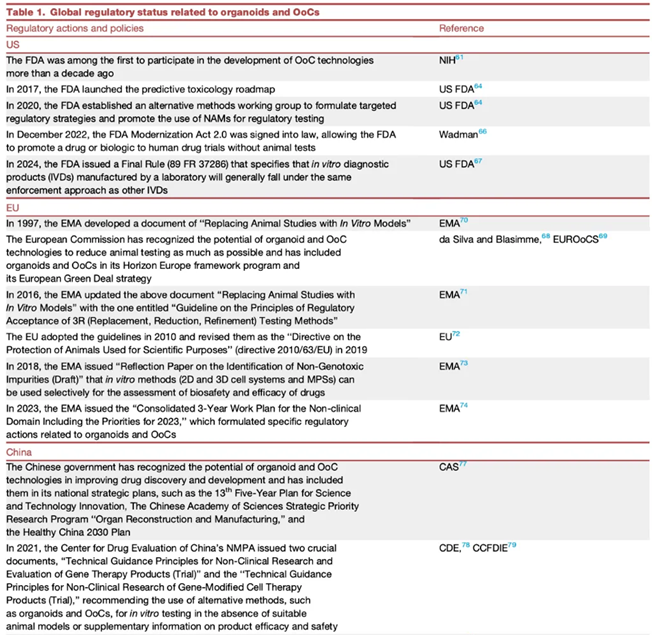
In terms of U.S. regulatory policies, in 2022, the FDA Modernization Act 2.0 was passed, which no longer mandates animal testing for new drugs. In December 2024, the U.S. Senate passed the FDA Modernization Act 3.0, aimed at reducing unnecessary animal testing while promoting scientific innovation, utilizing models such as organoids and organ-on-chip technologies to replace traditional animal testing. In April 2025, the FDA released the “Roadmap to Reducing Animal Testing in Preclinical Safety Studies.”
In terms of Chinese regulatory policies, in December 2021, the CDE (National Medical Products Administration) included organoids in the guidelines for CGT assessment models, recommending the use of cell and tissue-based models such as organoids to provide useful supplementary information for the evaluation of the efficacy and safety of cell gene therapy (CGT). In 2024, the CDE’s “Technical Guidelines for Non-Clinical Research of Human Stem Cell Products” and “Technical Guidelines for Non-Clinical Research of Tumor Therapeutic Vaccines” included organoids and organ-on-chip technologies as important sources of non-clinical research data. In January 2025, the CDE announced support for innovation in cosmetic raw materials, deepening research on alternatives to animal testing methods, and accelerating their application in the safety assessment of new materials.
In terms of European regulatory policies, in 2016, the European Medicines Agency (EMA) revised the “3R (Replacement, Reduction, Refinement) Testing Methods Guidelines”: encouraging pharmaceutical companies to submit organoid data as a supplement or alternative to animal testing, especially for toxicity testing and the development of tumor therapeutic vaccines. In July 2024, Europe released the “Standardization Roadmap for Organ-on-Chip Technologies,” planning to establish a standardized system for organ-on-chip technologies by 2025 to promote technological validation and industrialization [2].
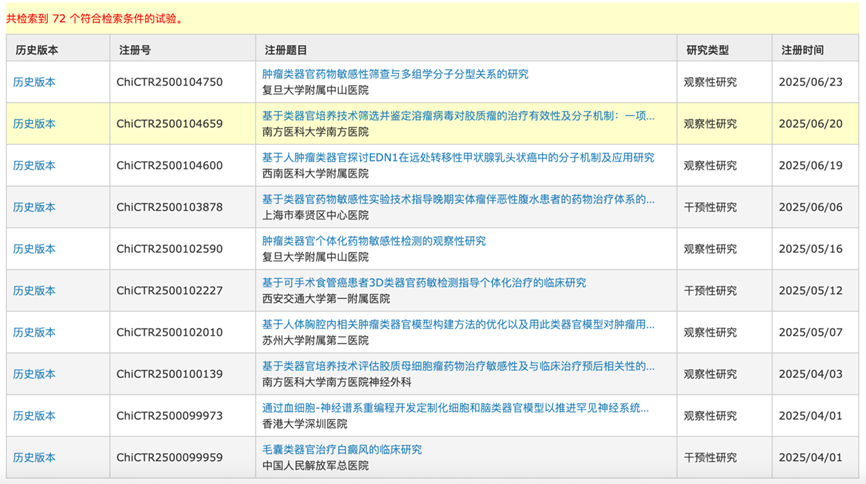
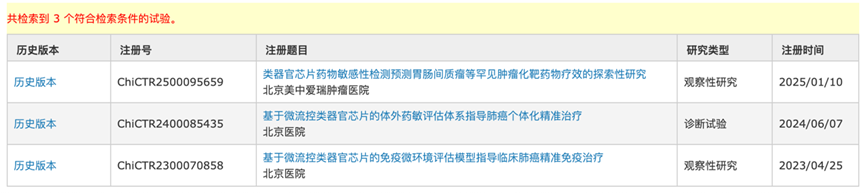
With the changes in regulatory policies, clinical trials related to organoids and organ-on-chip technologies are also continuously increasing. According to the China Clinical Trial Registration Center, from 2017 to June 2025, a total of 72 registered clinical trials related to organoids have been conducted, including observational studies, interventional studies, basic scientific research, and etiological studies.
From 2023 to June 2025, there have been 3 clinical trials related to organ-on-chip technologies, mainly related to tumor drug sensitivity testing, conducted by Beijing Hospital and Beijing Meizhong Aier Oncology Hospital.
3. Continuous Enrichment of Products and Services — Analysis of Domestic and International Companies Related to Organoids and Organ-on-Chip Technologies
The main products offered by companies related to organoids and organ-on-chip technologies include complete reagent kits for organoids, normal tissue organoid kits, tumor organoid kits, single organ chips, combination organ chips, and organoid culture equipment. The main services provided by these companies include CRO services for new drug development using organoids, tumor drug sensitivity testing services, organoid biobank construction services, and organoid regenerative medicine research services.
At the beginning of the industry development, Professor Hans Clevers’ team in the Netherlands cultivated the first intestinal organoid from adult stem cells derived from mouse intestines in 2009, thus opening a new era of organoid research. Based on years of accumulated research results, Hans Clevers founded the organoid technology incubation company HUB Organoids in 2013. HUB owns a foundational patent portfolio for organoids and provides services ranging from new model generation to analysis development and high-throughput screening, which has also led to the emergence of several organoid companies.
Subsequently, overseas life science product companies such as Thermo Fisher, Danaher, Stemcell, and Agilent provided organoid-related reagent kits and other products to domestic research institutions and enterprise users. With the impact of the U.S.-China trade war, the COVID-19 pandemic, and tariff wars, Chinese organoid/organ-on-chip companies that provide high-cost performance products and high-quality localized services have rapidly developed. For example, Bozhen Bio and other organoid companies have also developed numerous organoid-related reagent kits and equipment products through breakthroughs in upstream core technologies and patent layouts.
Chinese companies have also leveraged domestic sample sizes and industrial manufacturing advantages to develop a richer product pipeline and automated culture validation platforms, gradually moving the organoid/organ-on-chip industry towards high-throughput, automation, and standardization. In recent years, many organoid/organ-on-chip companies have also received significant financing.
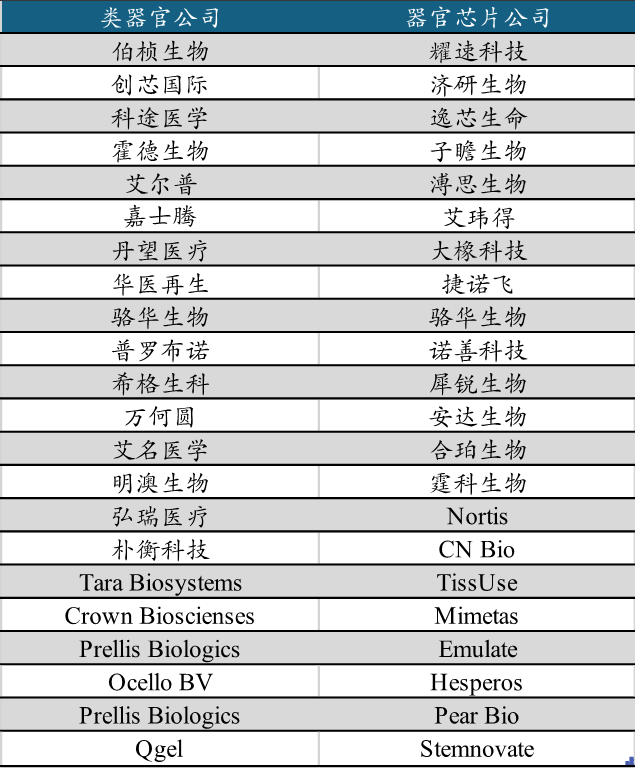
Meanwhile, numerous mergers and acquisitions of overseas organoid/organ-on-chip companies have also emerged. Pharmaceutical companies, life science companies, CROs, and AI pharmaceutical companies are frequently laying out in the organoid/organ-on-chip field: organoid representative company HUB Organoids was acquired by Merck at the end of 2024, organ-on-chip company Nortis was acquired by Quris AI in 2024, organoid company Cellesce was acquired by Molecular in 2022, Novoheart was acquired by Medera in 2020, and Crown Biosciences was acquired by JSR Co in 2018.
The main customer types for organoid/organ-on-chip companies include: pharmaceutical companies, CROs, IVD companies, hospitals, and research institutions. Application directions mainly include: empowering new drug development (toxicity prediction, CGT therapy evaluation, screening targeted drugs for specific mutation tumor organoids); tumor drug sensitivity testing (precision medication for cancer patients), cosmetic testing (humanized functional validation); organoid biobank construction (for research on the mechanisms of infectious diseases, cancer, etc.); regenerative medicine research (liver regeneration, hair follicle regeneration, solid organ stem cell transplantation, organ repair) [3].
In 2022, Sanofi successfully simulated the attack process of a patient’s chronic inflammatory demyelinating polyneuropathy (CIDP) using the “neuromuscular chip” developed in collaboration with Hesperos, successfully advancing SAR445088 to FDA approval for clinical trials, becoming the world’s first new drug to gain clinical approval solely based on “organoid chip” data.
In 2023, Roche announced the establishment of the Human Biology Research Institute (IHB), focusing on advancing research in human model systems such as organoids to accelerate drug development. Prior to this, they also invited Professor Hans Clevers, the pioneer of organoids, to lead the drug research and early development department.
4. Opportunities for the Integration of AI with Organoids and Organ-on-Chip Technologies
The FDA document also states that AI predictive models are a new method that can effectively replace animal testing in the future, simulating drug metabolism pathways through algorithms to accelerate toxicity predictions, etc. The focus is on modeling based on real human data, which can predict safety, immunogenicity, and pharmacokinetics, thereby reducing the need for new animal testing.
In the future, the FDA encourages companies to submit NAMs data alongside animal data; if effective organoid or AI model validation results can be provided, only a minimal amount or even no animal testing will be required. At the same time, the FDA accepts real-world human usage data from international sources as a basis for new drug evaluations and gradually includes a comprehensive database of animal and human toxicity data.
The combination of organoids and AI is an emerging field in organoid research. Researchers are creating more complex and accurate human organ functions and disease models by integrating organoids with AI, providing powerful tools for drug discovery, disease diagnosis, and treatment development.
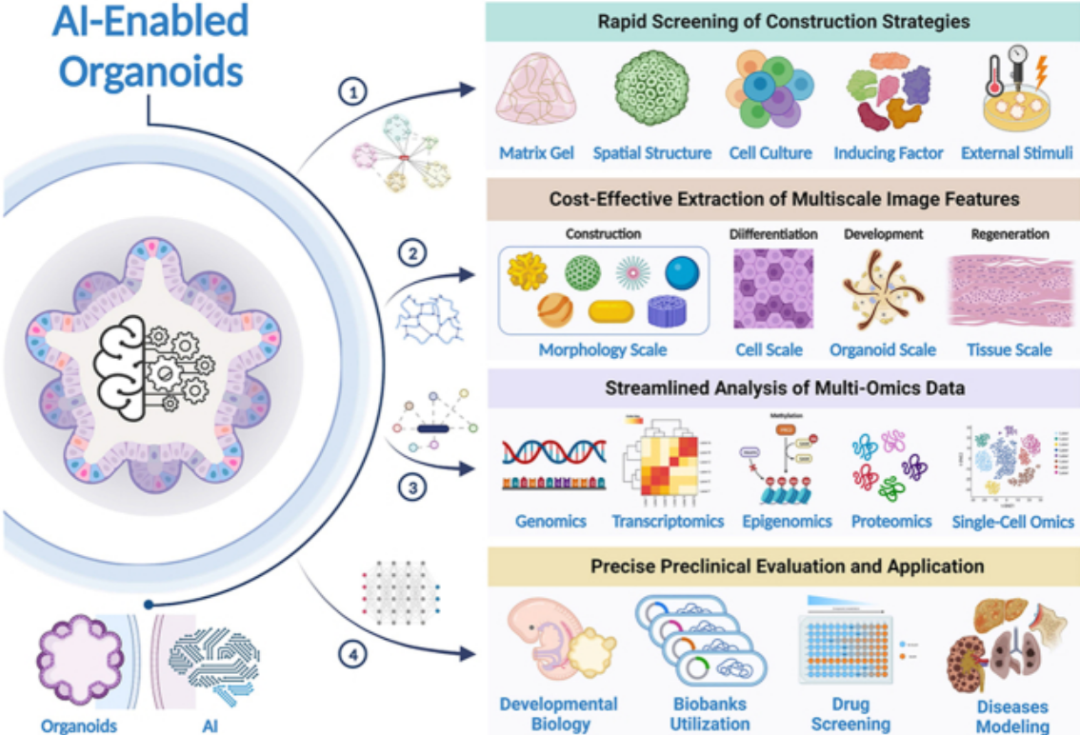
AI-assisted efficient construction of organoids: During the cultivation of organoids, machine learning algorithms can be combined with artificial intelligence to analyze large amounts of data, determine the most effective matrix gel synthesis methods, identify the spatial structure of the matrix gel, fine-tune cell culture conditions, identify active inducing factors, and assess external stimuli to achieve more efficient and higher quality organoid construction [4].
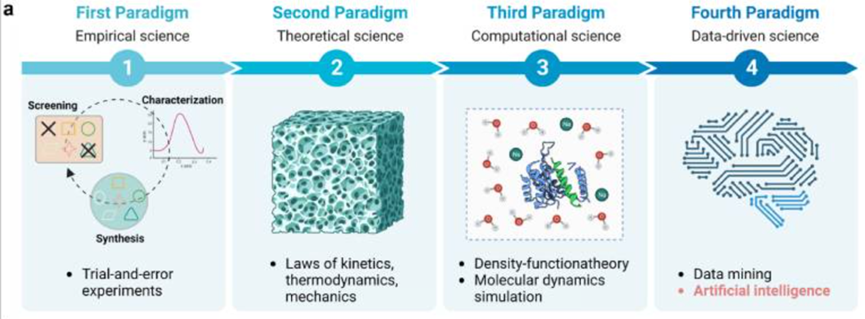
AI image analysis at the cellular scale related to organoids: In the evaluation of organoid functions, such as cell counting, cell morphology, and cell behavior, valuable information can be provided. Traditional analysis methods have significant drawbacks, including time-consuming manual counting, subjective bias, and the inability to accurately quantify complex cell features. The introduction of artificial intelligence can solve these problems and significantly improve the efficiency of organoid evaluations.
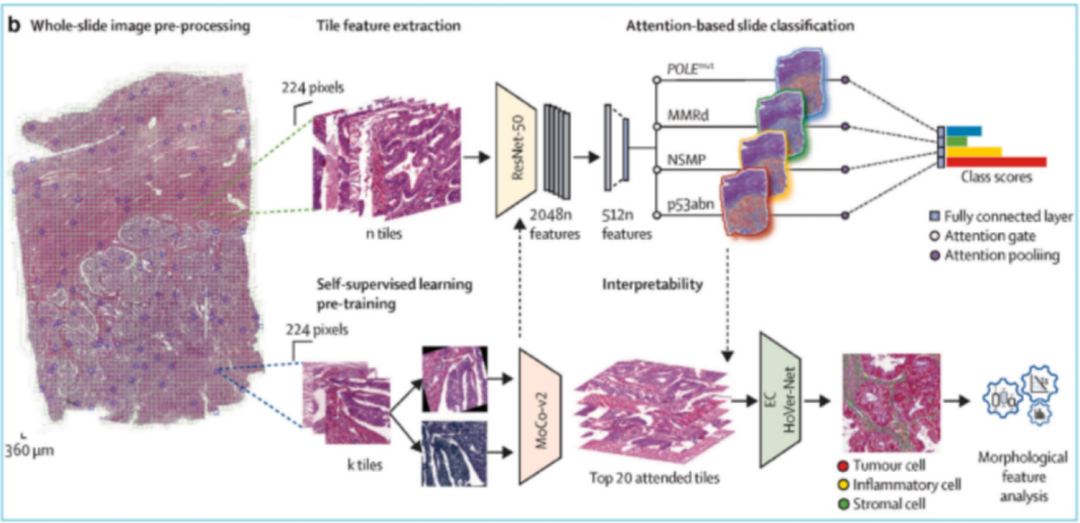
AI image analysis at the organoid level: Features of organoids, such as size, shape, internal structure, and the presence of specific organoid markers, can assist in the analysis of organoid function and maturity. Machine learning algorithms can be trained on large datasets of organoid-scale images to learn to recognize and quantify various organoid features. These algorithms can be used to analyze multiple organoid-scale images, providing rapid and accurate organoid analysis.
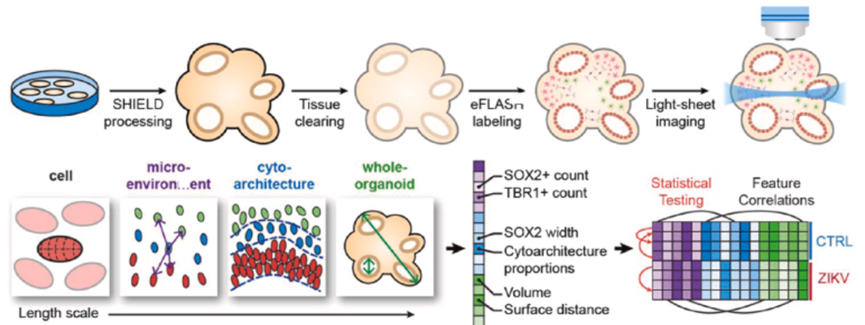
AI image analysis related to organoids at the tissue level: Evaluating tissue-level features is a key step in the field of organoid research and development, such as cellular organization, tissue structure, and specific tissue markers, which can provide information for the evaluation of organoid function and maturity. Artificial intelligence algorithms can be used to analyze large amounts of tissue-scale images, providing rapid and accurate tissue analysis, which can significantly improve the efficiency of organoid evaluations, reduce the time and effort required for manual analysis, and is expected to transform rapid diagnostic assessments during surgical procedures and enhance image quality.
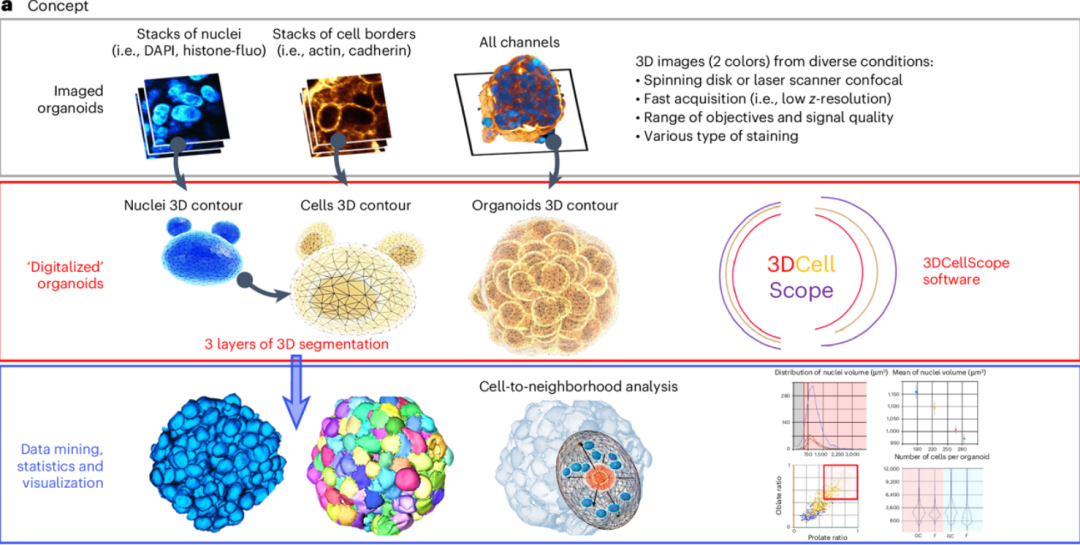
In 2025, Nature Methods published a groundbreaking study on AI and organoids, proposing a multi-layer segmentation and cellular topology analysis process based on AI, achieving full-dimensional digital analysis of organoids from subcellular to overall structures. Due to the intricate interactions of cells within organs and tissues, forming unique 3D tissue (tissue patterns) layers, tubes, or complex clusters, which are crucial for normal organ function. The 3DCellScope system and a comprehensive toolset for screening 3D organoid models demonstrate the broad application potential of AI and organoids in biomedical research [5].
Organoids/organ-on-chip technologies can also accumulate a large amount of interaction data during drug development and drug sensitivity testing processes. By extensively utilizing organoids, high-quality databases can be generated. Due to the data types including unstructured, semi-structured, and heterogeneous architectures with various features, as well as the complex relationships between omics data involving linear and nonlinear relationships, traditional analysis methods show significant limitations. After integrating artificial intelligence, analyzing high-throughput multi-omics data can accelerate the transition of organoids/organ-on-chip technologies from laboratory research to clinical applications. Through predictive models and optimization algorithms, the value of organoids in the drug development process can be efficiently assessed, including studying related mechanisms, screening potential drugs, and constructing in vitro disease models, etc.
[1] FDA, Roadmap to Reducing Animal Testing in Preclinical Safety Studies
[2] Zhou L, Huang J, Li C, Gu Q, Li G, Li ZA, Xu J, Zhou J, Tuan RS. Organoids and organs-on-chips: Recent advances, applications in drug development, and regulatory challenges. Med. 2025 Apr 11;6(4):100667. doi: 10.1016/j.medj.2025.100667. PMID: 40220744.
[3] Corrò C, Novellasdemunt L, Li VSW. A brief history of organoids. Am J Physiol Cell Physiol. 2020 Jul 1;319(1):C151-C165. doi: 10.1152/ajpcell.00120.2020. Epub 2020 May 27. PMID: 32459504; PMCID: PMC7468890.
[4] Bai L, Wu Y, Li G, Zhang W, Zhang H, Su J. AI-enabled organoids: Construction, analysis, and application. Bioact Mater. 2023 Sep 16;31:525-548. doi: 10.1016/j.bioactmat.2023.09.005. PMID: 37746662; PMCID: PMC10511344.
[5] Ong HT, Karatas E, Poquillon T, Grenci G, Furlan A, Dilasser F, Mohamad Raffi SB, Blanc D, Drimaracci E, Mikec D, Galisot G, Johnson BA, Liu AZ, Thiel C, Ullrich O; OrgaRES Consortium; Racine V, Beghin A. Digitalized organoids: integrated pipeline for high-speed 3D analysis of organoid structures using multilevel segmentation and cellular topology. Nat Methods. 2025 May 14. doi: 10.1038/s41592-025-02685-4. Epub ahead of print. PMID: 40369245.

Author: Hou Zhancai

Share

Like

View