 Humidity Disrupts Structural and Chiroptical Properties of Chiral 2D PerovskitesOriginal link:https://pubs.acs.org/doi/full/10.1021/acsnano.5c00480Keywords:Chiral 2D Perovskites;Humidity Degradation;Chiroptical Properties;Phase Transition;DFT Calculations
Humidity Disrupts Structural and Chiroptical Properties of Chiral 2D PerovskitesOriginal link:https://pubs.acs.org/doi/full/10.1021/acsnano.5c00480Keywords:Chiral 2D Perovskites;Humidity Degradation;Chiroptical Properties;Phase Transition;DFT Calculations
01
—
Overview
Chiral two-dimensional organic-inorganic hybrid metal halide perovskite semiconductors have emerged as a highly promising material platform for spintronic applications. However, it remains unclear how these materials change in crystal structure and chiroptical properties when exposed to atmospheric humidity. We found that when exposed to humidity, chiral 2D perovskites undergo phase transition degradation, forming one-dimensional (MBA)PbI3 (MBA = methylbenzylammonium) and (MBA)3PbI5·H2O hydrated phases, while the chiral response weakens or even disappears. First-principles simulations indicate that water molecules preferentially locate at the interface between the organic cations and the inorganic framework, disrupting hydrogen bonds and affecting the structural chirality and stability of the material. These findings provide important insights into the phase transition degradation mechanism of chiral 2D perovskites and its impact on chiral activity.
02
—
Research Background
Low-dimensional (especially two-dimensional) organic-inorganic hybrid metal halide perovskite (2D MHP) semiconductors have attracted significant attention due to their unique properties. These materials consist of well-defined inorganic metal halide layers and large organic spacer cations stacked alternately, forming a Ruddlesden-Popper (RP) crystal structure. 2D MHPs exhibit strong absorption, tunable wide bandgaps, compositional and structural diversity, and strong excitonic and dielectric constraints. Recent studies have shown that introducing chiral organic molecules into 2D MHPs can create chiral semiconductors that can control light, charge, and spin without an external magnetic field. Chirality describes an object that cannot be superimposed on its mirror image through any combination of rotation and translation, resulting in structures that have the same composition but different geometries.The first reported chiral 2D MHP material R-/S-/rac-MBA2PbI4 (R-/S-/rac-MBAPI) is based on chiral organic cations R- and S-α-methylbenzylammonium, referred to as R/S-MBA. Chiral 2D MHPs exhibit photochemical (photoactive) properties due to the interaction of the chiral structure with light, leading to different responses to left-handed and right-handed circularly polarized light. For example, R-/S-MBAPI exhibits circular dichroism (CD), which is the difference in absorption of left-handed and right-handed circularly polarized light. Charge transport in chiral structures depends on the spin configuration of the charge, which can be controlled in the absence of a magnetic field. Given these characteristics, chiral materials have various potential applications in optoelectronics, quantum computing, and spintronics.Most MHP materials degrade in the presence of humidity. Therefore, studying the humidity-induced degradation of novel optoelectronic materials such as chiral 2D perovskites is crucial. Compared to “ordinary” 2D MHPs, chiral counterparts exhibit inherent structural instability. This instability arises from the steric hindrance of the CH3 group at the α position against the polar head of NH3+. With few exceptions, the CH3 group is located at the β position of the NH3+ group, while most chiral 2D perovskites exhibit steric hindrance at the α position. This steric repulsion reduces the penetration depth of the NH3+ group at the inorganic sublattice cation sites. Consequently, due to the Coulomb interaction and weakened hydrogen bonds between the polar head of NH3+ and the axial halide anions, intrinsic instability in the material typically favors the formation of low-dimensional phases. This situation occurs for any chiral cation with CH3 at the α position of NH3+, as demonstrated in several chiral 2D perovskites: α-methylbenzylammonium, para-methoxy-α-methylbenzylammonium, 1-(1-naphthyl)ethylammonium, and 1-(2-naphthyl)ethylammonium. Based on this understanding, we investigated the effect of humidity on the structure and photochemical properties of R-/S-/rac-MBAPI.
03
—
Research Approach
Here, we report the effect of humidity on the model structure of R-/S-/rac-MBAPI and its chiroptical properties. Interaction with water molecules leads to the formation of one-dimensional (R-/S-MBA)PbI3 and hydrated phases. This phase transition degradation is accompanied by a weakening and disappearance of the CD signal. If a hydrated phase is formed, the two-dimensional phase can be reversibly restored through re-annealing; however, if a one-dimensional (MBA)PbI3 phase is formed, the phase transition is irreversible. DFT calculations indicate that water molecules preferentially locate at the interface between the organic cations and the inorganic framework, disrupting hydrogen bonds and affecting the chirality and stability of the chiral phase. Finally, grazing incidence wide-angle X-ray scattering (GIWAXS) measurements show that at 70% relative humidity (RH), degradation occurs through the formation of a one-dimensional phase after exposure to the same-handed opposite reflection phase. Interestingly, GIWAXS depth analysis indicates that degradation begins at the material/substrate interface and grain boundaries, rather than at the film surface. Our findings contribute to a deeper understanding of the correlation between chiral 2D MBAPI, humidity, and chiral activity. This knowledge is expected to have general relevance for the processing of chiral 2D perovskites that exhibit steric hindrance at the α position of the NH3+ group.
04
—
Research Content
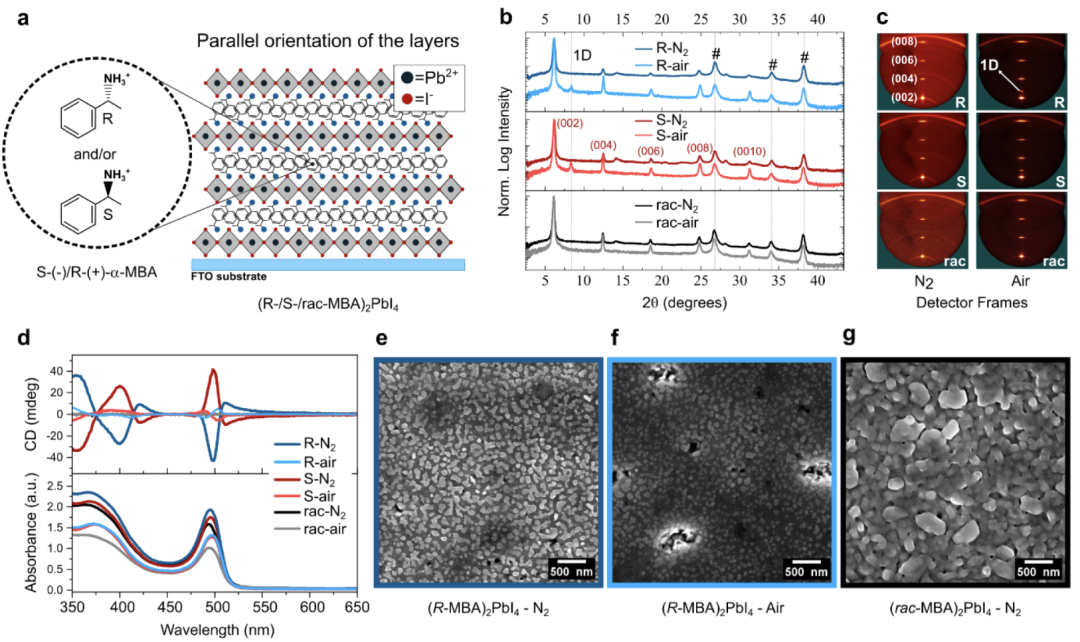
Two-dimensional R-/S-/rac-MBAPI films were synthesized starting from single crystals, followed by spin-coating deposition and annealing. These films were prepared from acetonitrile solutions of single crystals in a glove box filled with N2 or in ambient atmosphere (approximately 53% RH). The X-ray diffraction (XRD) patterns of R-/S-/rac-MBAPI films prepared in different environments are shown, along with the corresponding two-dimensional detector images. The XRD data indicate that the films prepared in an N2 atmosphere are phase-pure, with strong parallel orientation of the two-dimensional layers, evident from the strong out-of-plane Bragg reflections in the diffraction frames. The peak positions match the reference patterns calculated from single crystal data. The films prepared in ambient atmosphere also show oriented diffraction peaks, but R-/S-MBAPI exhibits additional one-dimensional phase impurities; namely, (R-/S-MBA)PbI3. The appearance of the one-dimensional phase may indicate that water interacted with the solvent/material during film formation, leading to a thermodynamically more stable one-dimensional phase.
Chiroptical responses of the materials were characterized through circular dichroism (CD) measurements. These highly parallel-oriented films may exhibit linear dichroism and linear birefringence (LDLB) due to possible macroscopic anisotropy (such as sample orientation-dependent absorption and different refractive indices along different crystallographic axes). To account for these effects, CD data were recorded from both the front and back sides of the films, and the real CD (CDgen) response and absorption g-factor were calculated. As expected, R-/S-MBAPI films show differences in absorption of circularly polarized light, while rac-MBAPI shows a flat line, indicating equal absorption regardless of polarization, consistent with previous reports. The CD peak positions of R-MBAPI and S-MBAPI are the same but with opposite signs, but the peaks of the air samples shift relative to those of the samples prepared in N2 at the excitonic transition. Although the excitonic absorption positions of the air and N2 R-/S-MBAPI films are roughly the same, the inflection points that produce chiral responses due to the Cotton effect shift to lower energy in the air samples. The microstructures of the N2 and air R-MBAPI films are relatively similar, while rac-MBAPI shows similarly sized grains but significantly larger sizes, as shown by SEM. While all films exhibit pinholes in the SEM images, it is noteworthy that the R-MBAPI prepared in air shows bright craters along the edges of the film, possibly due to the reaction of the two-dimensional material with atmospheric water. This result is consistent with the diffraction of R-MBAPI films. Cross-sectional SEM images show dense and smooth films, with thicknesses of approximately 360 nm for R-MBAPI and 300 nm for rac-MBAPI.
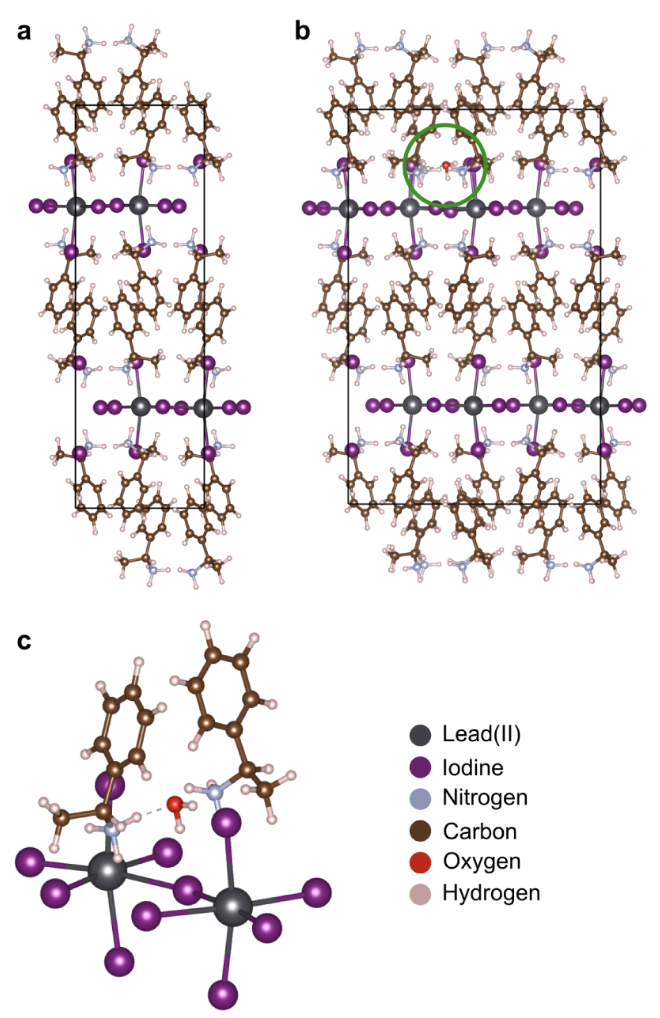
Next, we explore the impact of ambient atmosphere on the atomic-level details of chiral perovskites. In particular, we investigate the effect of H2O on the chirality of the R-MBAPI structure using density functional theory (DFT) calculations. Simulations indicate that water molecules preferentially adsorb at the interface between the chiral organic cation (R-MBA+) and the inorganic framework ([PbI6]4–). Here, H2O can form strong hydrogen bonds with the NH3+ head group of the cation (N–H···O) and the I– halide anion (O–H···I). In this way, water molecules disrupt the hydrogen bonds between the chiral organic cation and the inorganic framework, potentially facilitating the conversion of chiral 2D perovskites to one-dimensional or hydrated phases.
We then determine the effect of H2O molecules on the chirality of the chiral perovskite structure. To this end, we utilize the descriptor introduced by Pols et al. to describe the chiral distortion of the inorganic framework. In particular, we use this descriptor to characterize the structural chirality of the inorganic framework in the planar direction (𝜖∥MX4) by probing the helicity of the lead halide (Pb–I) bonds. Whenever H2O molecules embed into the structure, the structural chirality of the inorganic framework of R-MBAPI decreases (𝜖∥MX4 = −8.1 × 10–3 to 𝜖∥MX4 = −5.6 × 10–3). We speculate that the reduction in structural chirality of the inorganic framework (the source of the chiral signal) leads to a decreased degree of selective absorption of circularly polarized light. Combined with the changes in the perovskite phase after water adsorption (e.g., the conversion of the two-dimensional phase to one-dimensional or hydrated phases), these effects can explain the differences observed in CD measurements.
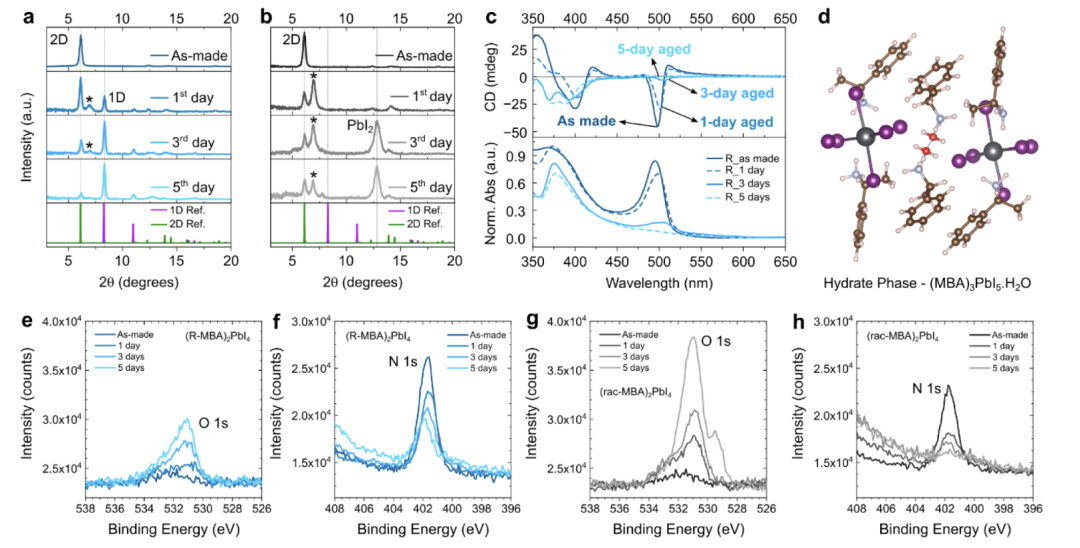
Next, we investigate the effects of aging under environmental conditions (approximately 53% RH) on the crystal structure and chiroptical response. The XRD patterns of fresh and aged R-MBAPI and rac-MBAPI initially show peaks belonging to phase-pure two-dimensional phases, which subsequently lose their two-dimensional structure at the expense of one-dimensional, hydrated, or PbI2 phases. Interestingly, the degradation of the same-handed opposite reflection phase differs from that of the racemic phase. In the latter case, we observe the formation of PbI2 and hydrated phases, while in the same-handed opposite reflection material, one-dimensional and hydrated phases are formed.
We propose that when exposed to humidity in the dark, hydrated phases of R-/S-/rac-MBAPI are formed. This iodine-based hydrated phase has not been reported, but the hydrated structures of Br/Cl phases (for racemic materials) support our hypothesis. For Br/Cl hydrated phases, the calculated powder diffraction patterns show major diffraction (200) at 2θ = 6.06° and 6.13°, respectively. This indicates that the d-spacing of the hydrated phase is very similar to that of the two-dimensional phase. Analysis of the hydrated structure shows that water molecules disrupt the two-dimensional layers but do not significantly alter the bilayer of MBA+ cations, maintaining a d-spacing close to that of the original two-dimensional phase. We hypothesize that a similar reaction occurs during the hydration of iodine-based materials, leading to the appearance of diffraction peaks at 2θ = 6.97°. Using Br hydrates as a template, we simulated the possible crystal structure of iodine-based hydrates. We observed that as larger halide anions are incorporated, the d-spacing increases, with major diffraction at 2θ = 5.99° (Figure S10, blue curve). Similarly, the d-spacing of this simulated structure supports the hypothesis of hydrate formation in the case of rac-MBAPI.
Like three-dimensional MHP films, water molecules penetrate into the perovskite structure and form hydrated perovskite phases. Generally, organic-inorganic hybrid perovskite crystals are characterized by ionic interactions between the two parts and hydrogen bonding interactions between the NH3+ polar part and halide anions. It is hypothesized that in CH3NH3PbI3, when water molecules form stronger hydrogen bonding interactions than with NH3+, hydrated phases are formed. This weakens the organic cation-PbI6 bonds, leading to the deprotonation of the organic cation and its volatilization as a neutral amine (e.g., methylamine). Additionally, water protonates the iodide, leading to the formation of volatile HI (hydroiodic acid). We hypothesize that water reacts in a similar manner, but the degradation mechanism is slightly different for racemic compared to R-MBAPI. For the same-handed opposite reflection phase, the hydrated phase appears to be an intermediate in the transition from the two-dimensional phase to the one-dimensional phase. This is evidenced by the rapid evolution of one-dimensional phase diffraction at 2θ = 8.3° and the weak diffraction of the hydrated phase at 2θ = 6.97°. In this case, the hydrated phase leads to the deprotonation of the MBA+ cation and the formation of HI, resulting in the formation of the one-dimensional phase.
For the racemic phase, the hydrated phase evolves at the expense of the two-dimensional phase, but is more persistent in the same-handed opposite reflection analog. Even after prolonged exposure, no one-dimensional phase diffraction was observed. After 3 days of exposure in air, PbI2 appears as a degradation phase. Therefore, for racemic materials, the hydration and proton exchange reactions seem to directly drive the formation of PbI2 from the two-dimensional phase: (rac−MBA)2PbI4(s)−→−H2OPbI2(s)+2MBA0(g)+2HI(g) We note that one-dimensional phase (rac-MBA)PbI3 has been reported in the literature; however, under our experimental conditions, the results indicate that the degradation mechanism of 2D rac-MBAPI does not involve the formation of its one-dimensional phase.
We also tested the reversibility of humidity-induced phase transitions by exposing fresh films to ambient humidity for 1 day and then annealing in an N2 atmosphere. While the hydrated phase is reversible and disappears during annealing, the concentration of the one-dimensional phase (as the degradation phase of R-MBAPI) increases relative to the two-dimensional phase after aging and re-annealing. This observation indicates that the diffraction appearing at 2θ = 6.97° indeed comes from the hydrated phase. The formation of volatile MBA and/or HI prevents the reversibility of the one-dimensional phase to the two-dimensional phase.
Aging R-MBAPI films showed reduced CD intensity, disappearance of CD peaks, and changes in peak positions. The CD peak positions follow the excitonic absorption band visible at approximately 500 nm. As the excitonic absorption weakens, the CD response decreases. With the emergence of one-dimensional and hydrated phases, the CD response becomes weaker, and the films degrade significantly. Another piece of evidence for film degradation and thickness reduction due to mass loss is the stronger FTO diffraction in aged films compared to freshly prepared films. The disappearance of the CD signal at the excitonic absorption supports our hypothesis that water molecules disrupt the inorganic sublattice, depleting the excitonic states, which are characteristic of the corner-sharing [PbI6]4– octahedra in the two-dimensional framework.
Surface-sensitive X-ray photoelectron spectroscopy (XPS) measurements were performed on R- and rac-MBAPI samples to assess the elemental composition of the surface. The table summarizes the relative elemental composition of the films normalized to the Pb 4f signal, assuming that Pb2+ species are least likely to leave the film or migrate in the studied materials. While fresh films show negligible O 1s signals, these increase after exposure to humidity and oxygen. The main difference is in the N 1s signal. For rac-MBAPI, it nearly disappears, while for R-MBAPI, it decreases only slightly. This is consistent with the aforementioned XRD results, as in the R-MBAPI samples, we primarily observed the formation of the one-dimensional phase, indicating only partial N loss. In the rac-MBAPI samples, we found a significant amount of PbI2, indicating that the organic molecules were almost completely lost, leading to a substantial reduction in the N 1s signal.
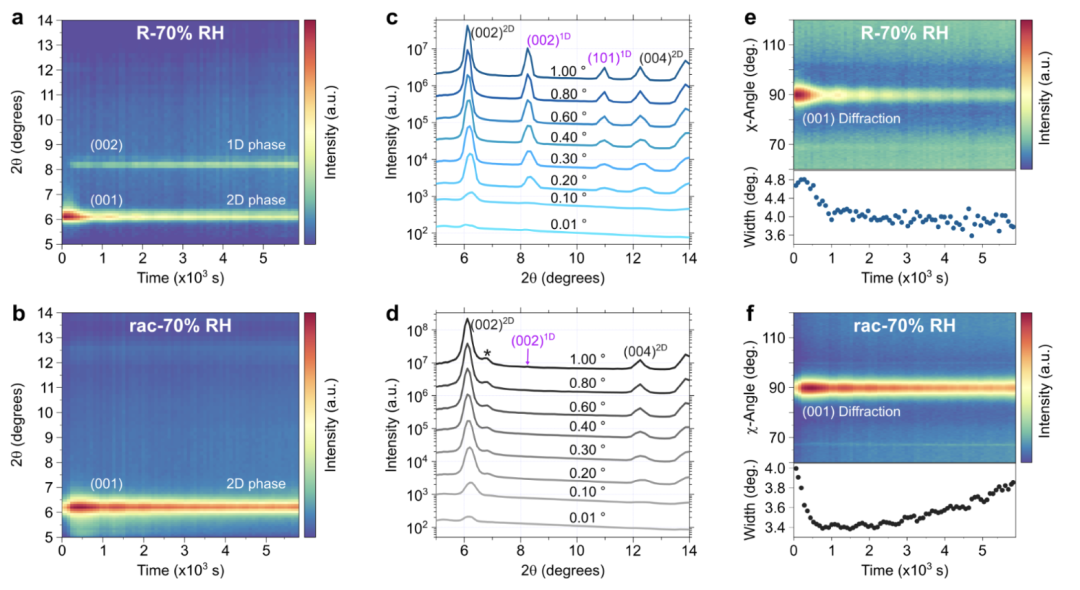
To evaluate the location of 2D phase decomposition during degradation and the changes in the thickness of the entire film, we performed in situ GIWAXS measurements at 70% RH. Fresh R-/rac-MBAPI films were loaded into a humidity chamber (RH < 1%) under a flow of N2; then the RH was increased to 70% while measuring GIWAXS at an incident angle α = 0.01° for 90 min. With this incident angle, we only probe a few nanometers below the surface (approximately 3 nm). The emergence of one-dimensional phase diffraction at the surface of R-MBAPI films reveals the high sensitivity of the same-handed opposite reflection phase to humidity. In contrast, the racemic phase shows no changes at the material surface other than a decrease in diffraction intensity. Ahn et al. verified that under controlled conditions (25°C and 20% RH), the same-handed R-/S-MBAPI films are quite stable, maintaining the same structural characteristics for 7 days. Ma et al. found no changes in the diffraction patterns of aged single crystals after 67 days. In contrast, our experiments reveal the high sensitivity of the same-handed and rac-MBAPI films to humidity, with the emergence of one-dimensional or hydrated phases occurring within a day in ambient atmosphere (approximately 53-54% RH) and within minutes at 70% RH. Ishii and Miyasaka tested the thermal and environmental stability of (R/S-1NEA)2PbBr4 and (R/S-2NEA)2PbBr4, where 1/2NEA are 1- and 1-(2-naphthyl)ethylammonium cations, respectively. Both chiral 2D MHPs maintained good structural stability under environmental conditions for several weeks. However, at 75% relative humidity (RH) and 75°C, (R/S-1NEA)2PbBr4 lost its structure and CD response, also forming a one-dimensional phase, while (R/S-2NEA)2PbBr4 maintained its two-dimensional structure for a longer time due to stronger hydrogen bonding interactions. Similar chiral materials have also reported this hydration sensitivity. For example, Dučinskas et al. found that Dion-Jacobson phase two-dimensional perovskites are also very prone to hydration. Interestingly, the dication species structure based on 1,4-benzenediammonium also forms one-dimensional phases upon hydration. This hydration is reversible but requires additional annealing steps at the cost of crystallinity and lower photoluminescence intensity.
To verify the internal changes of the materials, we measured GIWAXS at different incident angles from α = 0.01 to 1.0° after humidity exposure. Notably, both films (R-/rac-MBAPI) exhibit nearly pure two-dimensional phases after aging. Only with increased incident angles (α > 0.1°) do the hydrated and one-dimensional phases become more apparent. Similarly, the same-handed opposite reflection phase tends to form one-dimensional phases, while the racemic phase tends to form hydrated phases. The peak intensities of the impurity phases increase with increasing incident angle, indicating that the degradation of the two-dimensional phase does not begin at the film surface.
For three-dimensional MHP solar cells, it has been reported that degradation under environmental conditions and solar spectrum irradiation may begin at the electron or hole transport layer/perovskite interface. The degradation of chiral 2D MHPs is more likely to start from grain boundaries and the material-substrate interface rather than from the surface. This is attributed to the high hydrophobicity of the surface, which is terminated by MBA+ cations, as shown by time-of-flight secondary ion mass spectrometry (ToF-SIMS) analysis of two-dimensional perovskites. Additionally, all films exhibit pinholes, which may allow water molecules to reach more hydrophilic sites within the material. Throughout the degradation process, we did not observe significant changes in the film texture, as can be seen from the χ angle intensity distribution. The intensity of the diffraction rings peaks at 90°, i.e., in the out-of-plane direction. The full widths at half maximum of these peaks are very narrow (Δχ ∼ 4°), demonstrating the highly uniform planar texture of these materials. Therefore, achieving smooth surfaces, highly oriented crystalline films, and the preparation of films without pinholes may be effective strategies to enhance the stability of chiral 2D perovskite films.
StatementThis article is for academic sharing only. Images are sourced from screenshots of the above link; if there is any infringement, please contact for modification or deletion, thank you!