In flow cytometry detection, dead cells can easily lead to non-specific staining, resulting in incorrect results. Therefore, excluding dead cells is crucial. For a more detailed interpretation, please refer to the two previous articles:
Three Figures to Help You Understand Why Cell Viability Assessment is Important in Experiments
Flow Cytometry Viability Dyes: It’s Not Just About Life and Death
The identification of dead cells is generally achieved by excluding them using DNA-binding dyes, which take advantage of the increased membrane permeability of dead cells, allowing DNA dyes to enter without membrane rupture. However, the concentration and time used for these dyes are significantly lower than those used during the cell cycle, and fixation is not required.
However, for some intracellular antigens (cytokines, transcription factors), fixation and membrane rupture are required. Therefore, among the dyes mentioned above, except for some amine-based dyes (such as Live/Dead, Ghost, etc.) and the new anthraquinone dye CyTRAK Orange, other dyes are no longer suitable for cell viability assessment in such specimens.
PI Detection Channel: Use 488 nm excitation and collect with 610/20 BP.
DAPI Detection Channel: Ideally use 355 nm excitation and collect with 440/40 BP; 405 nm excitation and 450/50 BP collection can also be used.
7-AAD Detection Channel: Use 488 nm excitation and collect with 670/14 BP.
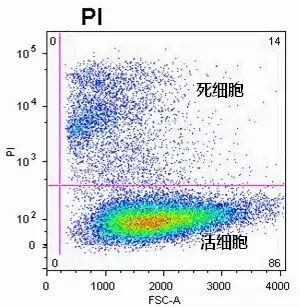
The image below shows the results of cell viability detection using PI: