Click the blue text
Follow us


Background Introduction

Hypoxia has been identified as a prominent feature within tumors, resulting from the rapid and aggressive growth of tumor cells and abnormal vascularization. Consequently, the low concentration of oxygen (O2) in the hypoxic tumor microenvironment significantly weakens O2-dependent physiological mechanisms and further reduces therapeutic efficacy, such as that of photodynamic therapy (PDT), which typically generates reactive oxygen species (ROS) to kill cancer cells through energy transfer between photosensitizers (PS) in the excited triplet state (3PS*) and surrounding O2. Moreover, conventional PDT can even exacerbate tumor hypoxia due to oxygen consumption and microvascular damage, diminishing overall antitumor effects.
To overcome hypoxia in tumor PDT, two strategies have been developed. One approach is to utilize photodynamic nanoplatforms to directly increase oxygen production within tumors, such as by delivering oxygen to the tumor or transporting enzymes to decompose endogenous hydrogen peroxide (H2O2) to generate oxygen to alleviate hypoxia. The other method involves co-encapsulating PS and chemotherapeutic agents into nanoplatforms using pH or hypoxia-responsive carriers to achieve a combination of PDT and chemotherapy. Although these methods have improved treatment efficacy for hypoxic tumors to some extent, they have not fully resolved the issue due to the poor O2 loading capacity of O2 carriers and the low concentration of H2O2 at tumor sites. Additionally, most of these nanoplatforms employ multi-component inorganic materials (even non-biodegradable materials) and multi-step manufacturing processes, which affect their further application in clinical practice. Furthermore, they often release/activate chemotherapeutic agents in normal tissues. Therefore, a more effective and specific in vivo hypoxic tumor treatment technology is of significant importance in both preclinical and clinical settings.
Recently, PS capable of releasing Type I ROS, including hydroxyl radicals (OH•), superoxide radicals (O2•−), and other O2-related radicals, have become increasingly promising. This is because Type I PS can overcome the O2-dependent limitations of Type II PS, which primarily generates singlet oxygen (1O2). Unfortunately, due to a lack of effective molecular design methods, reports on Type I PS are much less common. Additionally, the relatively severe side effects of PS remain a significant concern, as common PS adopt an “always-on” strategy, which can lead to severe phototoxic symptoms, including skin redness, burning sensations, and scabbing, requiring patients to avoid exposure to natural light for weeks after treatment. Moreover, most existing PS typically exhibit poor hydrophilicity, leading to severe aggregation in aqueous solutions, resulting in reduced photoactivity or even complete quenching. Therefore, seeking a more convenient and manageable strategy to develop hydrophilic PS with good hypoxia resistance and minimal side effects holds great potential for improving hypoxic tumor treatment in clinical practice, but it still presents considerable challenges.

Overview of the Full Text

In this study, the authors introduce the development of an octa-substituted zinc phthalocyanine (PcN8O, Figure 1) derivative with eight N-oxide groups. Encouragingly, PcN8O exhibits significant hydrophilicity. PcN8O can be directly dispersed in water, forming a uniform and stable nanosuspension system, with a concentration of up to 29 mg/mL. This makes PcN8O an ideal candidate for photostable agents that can be used directly under biological conditions without the need for organic solvents. Meanwhile, PcN8O can spontaneously assemble to form jointly stable nanoparticles (NanoPcN8O), which serve as hypoxia-responsive bioreductive phototherapeutics for solid tumor imaging and treatment. Specifically, NanoPcN8O can undergo simple bioreduction under hypoxic conditions, producing a product NanoPcN8 rich in electron-donating tertiary amine groups, which initiates Type I photodynamic reactions to generate O2•− while activating photothermal effects to produce substantial heat. Due to these unique properties, NanoPcN8O holds great potential as an effective phototherapeutic agent capable of overcoming tumor hypoxia. Furthermore, NanoPcN8O can be used for solid tumor imaging in mouse models via photoacoustic (PA) imaging. Ultimately, these advantages significantly enhance the therapeutic response in vivo and effectively inhibit tumor growth through the combined effects of Type I PDT and photothermal therapy (PTT). Notably, NanoPcN8O does not induce skin phototoxicity, a very common side effect associated with traditional “always-on” PS phototherapy. Therefore, the authors believe this study will provide new perspectives for the diagnosis and safe treatment of hypoxic tumors.
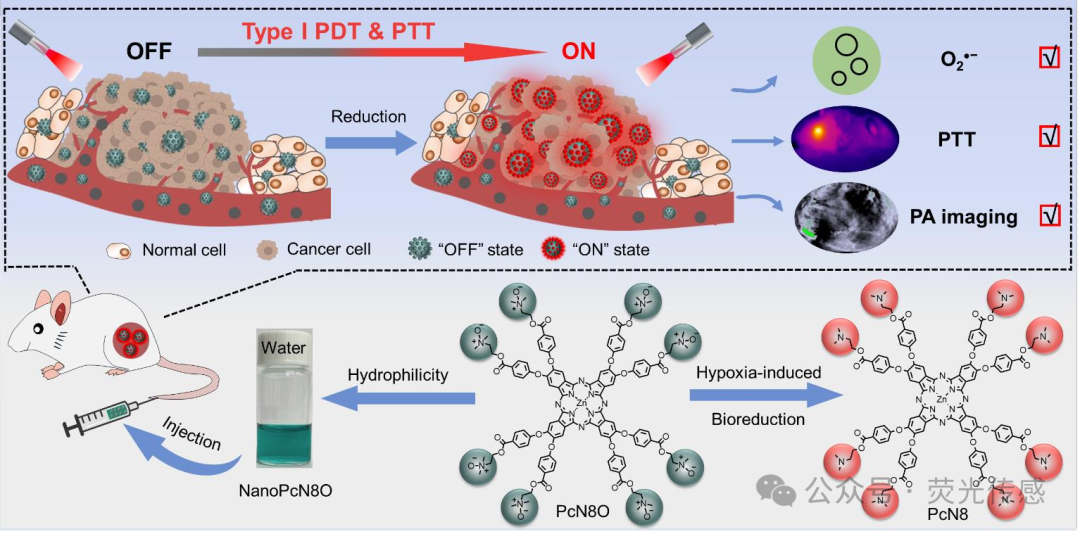
Figure 1. Schematic diagram of the design principle for hydrophilic phthalocyanine (PcN8O) bioreduction with N-oxide moieties in hypoxia-induced tumors, for specific Type I photodynamic and photothermal synergistic therapy with controlled “off-on” states.

Illustrated Guide

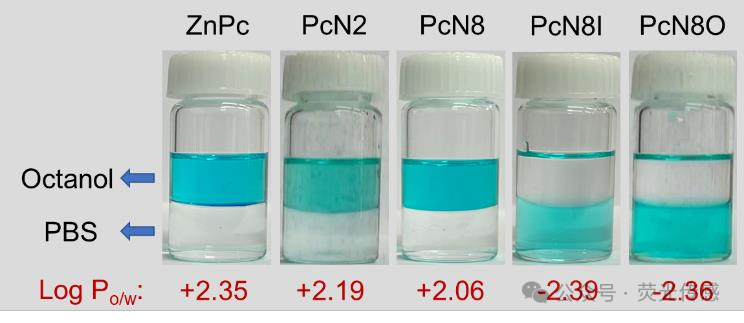
Figure 2. Hydrophilicity of Pcs, including ZnPc, PcN2, PcN8, PcN8I, and PcN8O. Photos of ZnPc, PcN2, PcN8, PcN8O, and PcN8I (all at 50 μM) mixed with octanol/PBS (v/v=1/1). All experiments were conducted at room temperature.
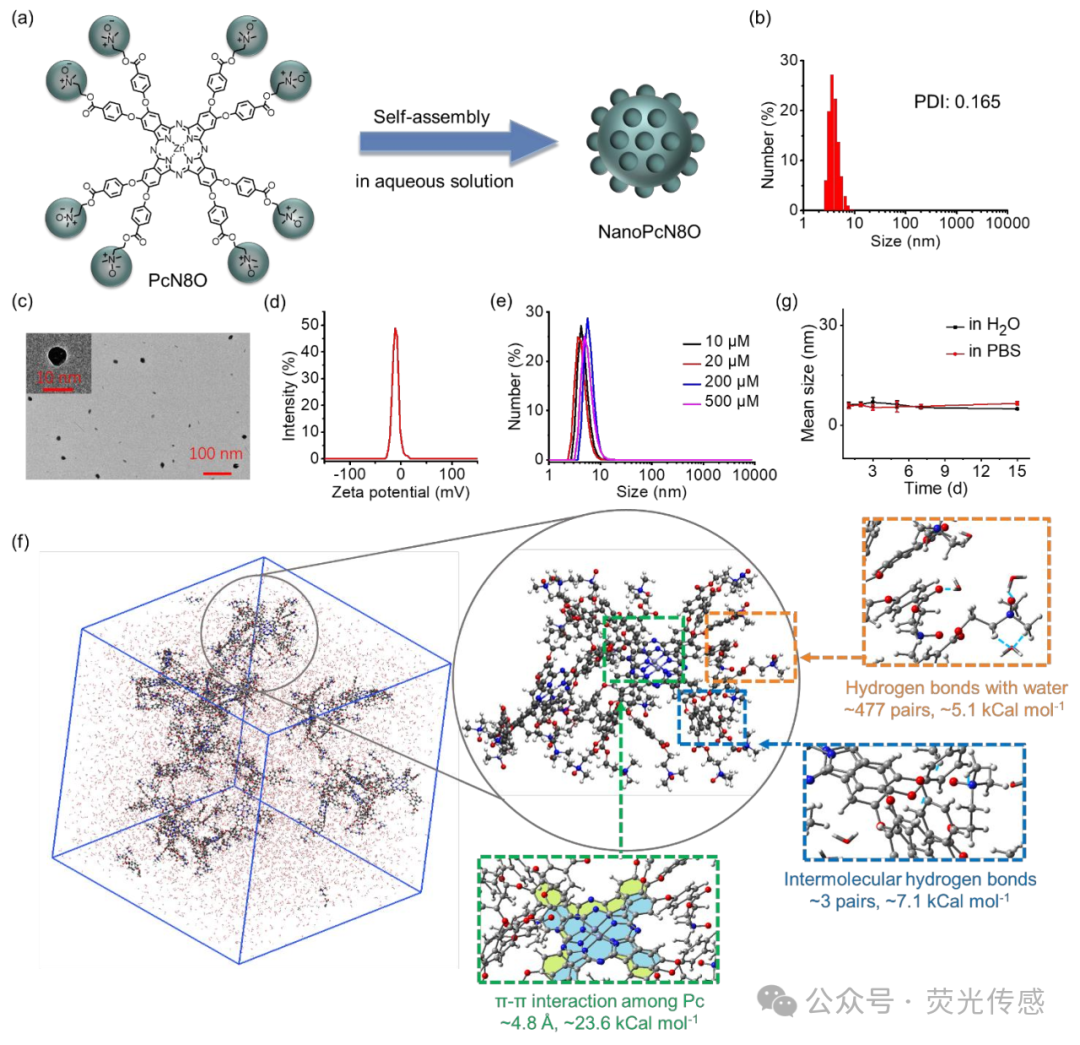
Figure 3. Preparation and characterization of nano-structured phthalocyanine assemblies. (a) Schematic diagram of the self-assembly of PcN8O to form NanoPcN8O. (b) Size distribution of NanoPcN8O (10 μM) in water detected by DLS. (c) Morphology of NanoPcN8O determined by TEM. (d) Zeta potential of NanoPcN8O (10 μM) in water. (e) DLS particle size distribution of NanoPcN8O at different concentrations. (f) Structure of the self-assembled system containing multiple PcN8O molecules obtained from MD simulations. The enlarged image shows representative structures, highlighting π-π interactions, hydrogen bonding with water, and intermolecular hydrogen bonding between PcN8O molecules. (g) Average particle size of NanoPcN8O (10 μM) in water and PBS detected by DLS after aging for different times.
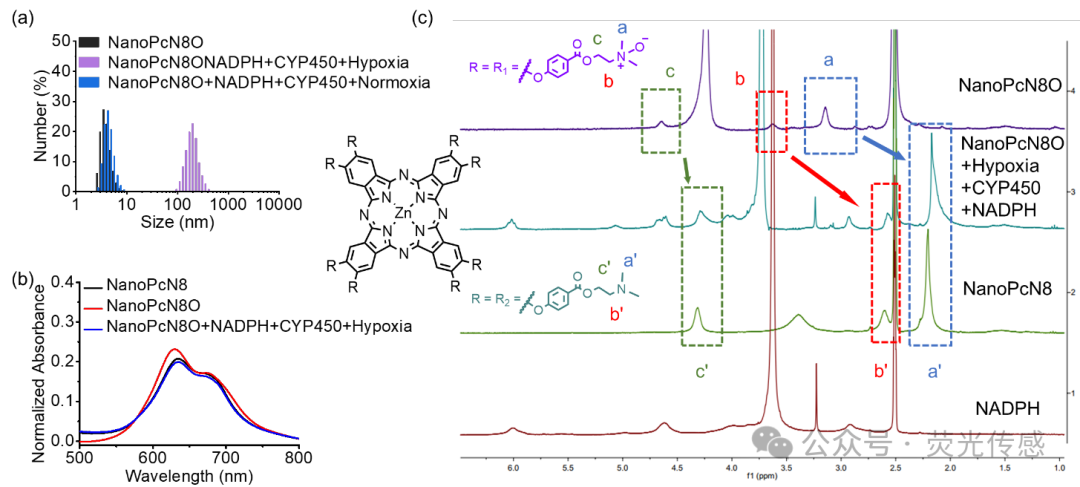
Figure 4. Hypoxia-induced bioreduction of NanoPcN8O. (a) Size distribution of NanoPcN8O dispersed in water after incubation with NADPH and CYP450 for 2 hours under normoxic and hypoxic conditions. The size distribution of NanoPcN8O in water is used as a control. (b) Absorption spectra of NanoPcN8O before and after incubation with NADPH and CYP450 in a hypoxic environment for 2 hours. NanoPcN8O is used as a control. (c) 1H NMR spectra of NanoPcN8O after incubation with NADPH and CYP450 under hypoxic conditions at 37°C for 2 hours. The 1H NMR spectra of NanoPcN8O and NADPH in DMSO-d6+D2O under normoxic conditions are used as controls. The 1H NMR spectra of NanoPcN8 under normoxic conditions in DMSO-d6 are used as controls.
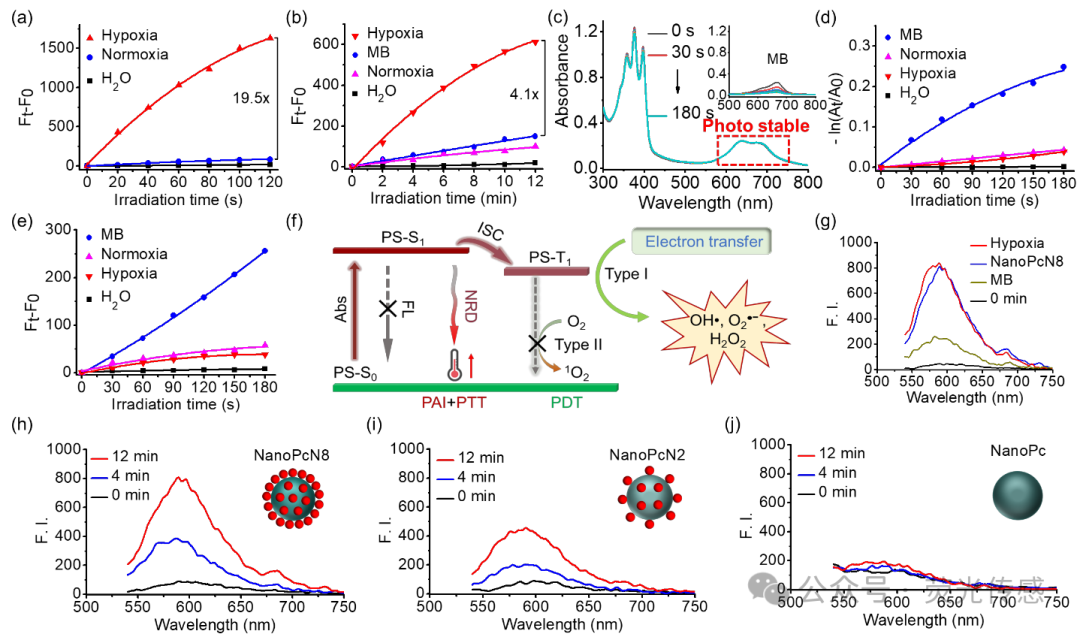
Figure 5. Photodynamic properties of NanoPcN8O. (a) ROS and (b) O2•− generation of NanoPcN8O (4 μM) after incubation with NADPH and CYP450 in water under normoxic and hypoxic conditions for 2 hours, detected using DCFH-DA and DHE as fluorescent probes, respectively. The aqueous solution of NADPH and CYP450 is used as a control. (c) Photodegradation curve of ABDA under 685 nm light irradiation (20 mW cm-2) in the presence of NanoPcN8O (insert: MB). (d) 1O2 generation of NanoPcN8O (4 μM) after incubation with NADPH and CYP450 in water under normoxic and hypoxic conditions for 2 hours, detected using ABDA as a detector. The aqueous solution of NADPH and CYP450 is used as a control. (e) 1O2 generation of NanoPcN8O (4 μM) after incubation with NADPH and CYP450 in water under normoxic and hypoxic conditions for 2 hours, detected using SOSG as a fluorescent probe. The aqueous solution of NADPH and CYP450 is used as a control. (f) Schematic diagram of O2•− generation induced by hypoxia in NanoPcN8O. Abs, absorption; FL, fluorescence; ISC, intersystem crossing; NRD, non-radiative decay; PDT, photodynamic therapy; PTT, photothermal therapy. (g) O2•− generation in water after 12 minutes of 685 nm light irradiation (20 mW cm-2) with NanoPcN8O (incubated with NADPH and CYP450 under hypoxic conditions for 2 hours), NanoPcN8, and MB (all at 4 μM). F.I., fluorescence intensity. Using DHE as a fluorescent probe, (h) O2•− generation can be promoted by (i) NanoPcN8, (j) NanoPcN2, and (k) NanoPc (all at 4 μM).
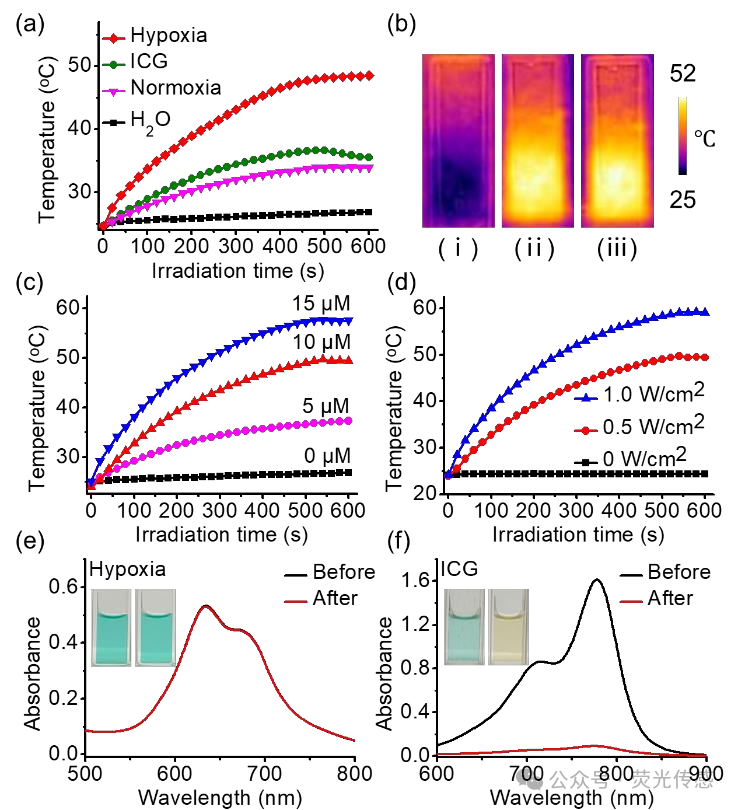
Figure 6. Photothermal properties of NanoPcN8O in water. (a) Time-dependent temperature of NanoPcN8O under normoxic and hypoxic conditions (10 μM) after incubation with NADPH and CYP450 in water for 2 hours, followed by 10 minutes of 685 nm light irradiation (0.5 W cm-2). The time-dependent temperature of ICG (10 μM) in water after 10 minutes of 808 nm light irradiation (0.5 W cm-2) is used as a control. (b) Photothermal imaging of NanoPcN8O (10 μM) in water under (i) normoxic and (ii) hypoxic conditions after 2 hours of incubation with NADPH and CYP450, followed by 10 minutes of 685 nm light irradiation (0.5 W cm-2). (iii) NanoPcN8O is used as a control. (c) Temperature increase of NanoPcN8O under hypoxic conditions after incubation with different concentrations (0, 5, 10, and 20 μM) of NADPH and CYP450 for 2 hours, followed by 10 minutes of 685 nm light irradiation at a fixed power density of 0.5 W cm-2. (d) Temperature increase of NanoPcN8O (10 μM) under hypoxic conditions after 10 minutes of 685 nm light irradiation at different power densities (0, 0.5, and 1.0 W cm-2), following 2 hours of incubation with NADPH and CYP450. (e) Absorption spectra of NanoPcN8O after incubation with NADPH and CYP450 under hypoxic conditions and (f) ICG before and after 10 minutes of 808 nm light irradiation (0.5 W cm-2). The insert shows photos of nanoPCN80 and ICG water suspensions before (left) and after (right) light irradiation.
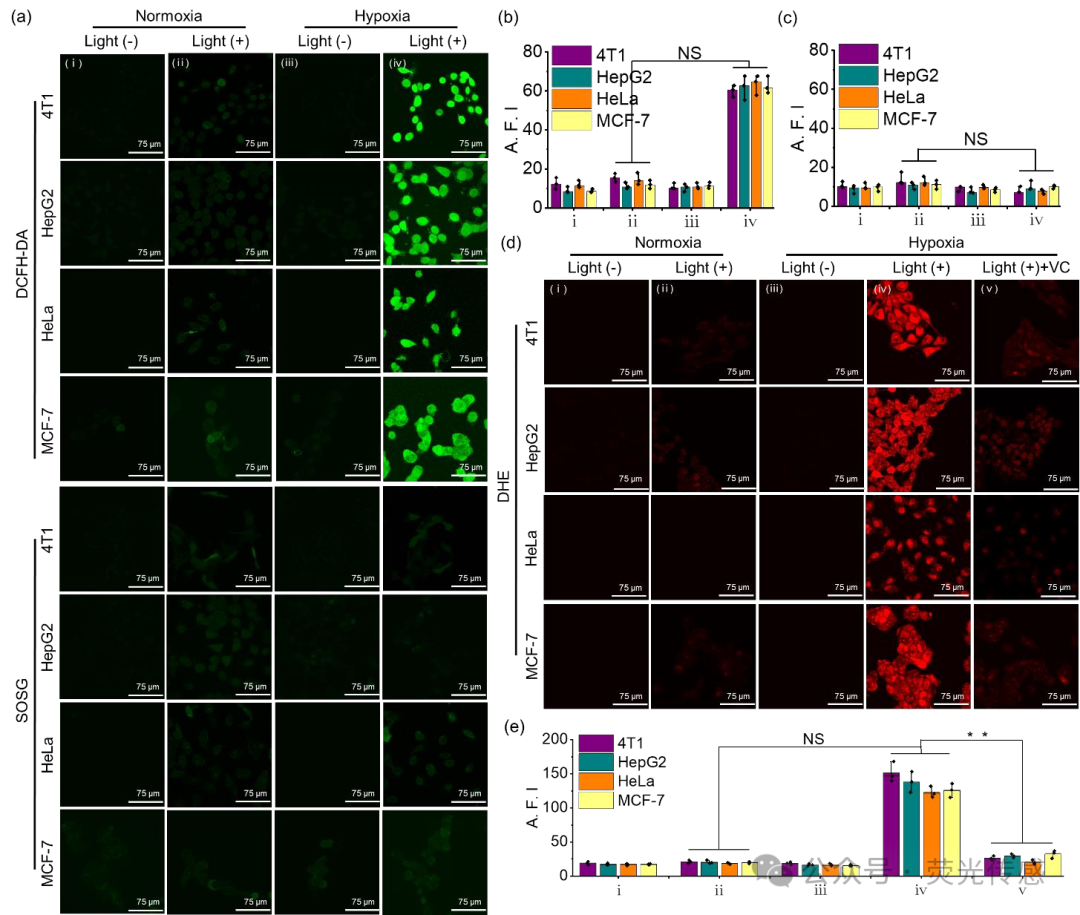
Figure 7. In vitro O2•− generation of NanoPcN8O under normoxic and hypoxic conditions. (a) Confocal laser scanning microscopy (CLSM) images of cellular ROS and 1O2 detection in NanoPcN8O-treated 4T1, HepG2, HeLa, and MCF-7 cells under (i) normoxic (no light), (ii) normoxic, (iii) hypoxic (no light), and (iv) hypoxic conditions after 60 seconds of 685 nm light irradiation (0.1 W cm-2) using DCFH-DA and SOSG as fluorescent probes, respectively. (b) Quantitative detection of intracellular (b) ROS and (c) 1O2 levels in NanoPcN8O-treated 4T1, HepG2, HeLa, and MCF-7 cells under (i) normoxic (no light), (ii) normoxic, (iii) hypoxic (no light), and (iv) hypoxic conditions after 60 seconds of 685 nm light irradiation (0.1 W cm-2). A.F.I., average fluorescence intensity. (d) CLSM images and (e) quantitative detection of intracellular O2•− levels in NanoPcN8O-treated 4T1, HepG2, HeLa, and MCF-7 cells under VC conditions after 60 seconds of 685 nm light irradiation (0.1 W cm-2) under (i) normoxic (no light), (ii) normoxic, (iii) hypoxic (no light), (iv) hypoxic, and (v) hypoxic conditions.
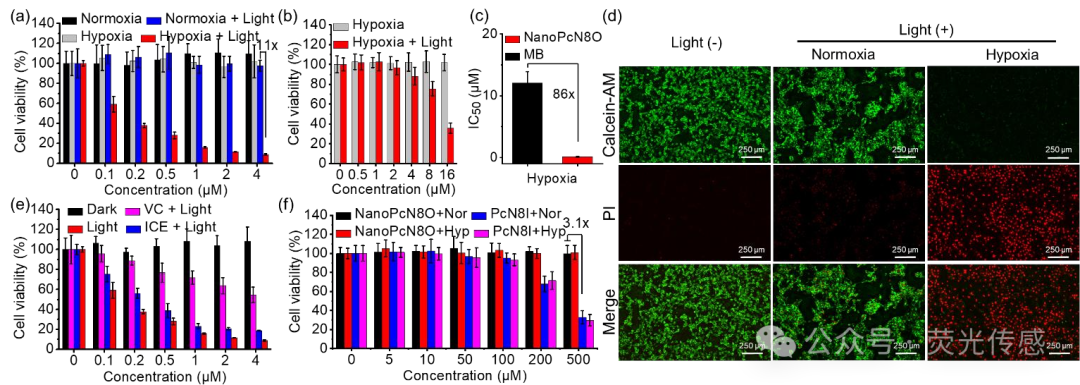
Figure 8. In vitro phototherapeutic effects of NanoPcN8O under normoxic and hypoxic conditions. (a) Cytotoxic effects of NanoPcN8O on 4T1 cells under normoxic or hypoxic conditions after 5 minutes of 685 nm light (0.5 W cm-2) irradiation. (b) Cytotoxic effects of MB on 4T1 cells under hypoxic conditions after 5 minutes of 685 nm light (0.5 W cm-2) irradiation. (c) Half-maximal inhibitory concentration of NanoPcN8O and Ce6 under hypoxic conditions. (d) Detection of calcein AM/PI co-staining by fluorescence microscopy (scale bar = 250 μm). Cells were divided into three groups: (i) normoxic (no light), (ii) normoxic + NanoPcN8O (4 μM) + 685 nm (0.5 W cm-2, 5 minutes), and (iii) hypoxic + NanoPcN8O (4 μM) + 685 nm (0.5 W cm-2, 5 minutes). The green channel shows calcein AM staining, and the red channel shows PI staining. (e) PDT (ice + 685 nm light) and PTT (VC + 685 nm light) effects of NanoPcN8O on 4T1 cells under hypoxic conditions. (f) Cytotoxic effects of NanoPcN8O and PcN8I on L929 cells under normoxic (Nor) and hypoxic (Hyp) conditions.
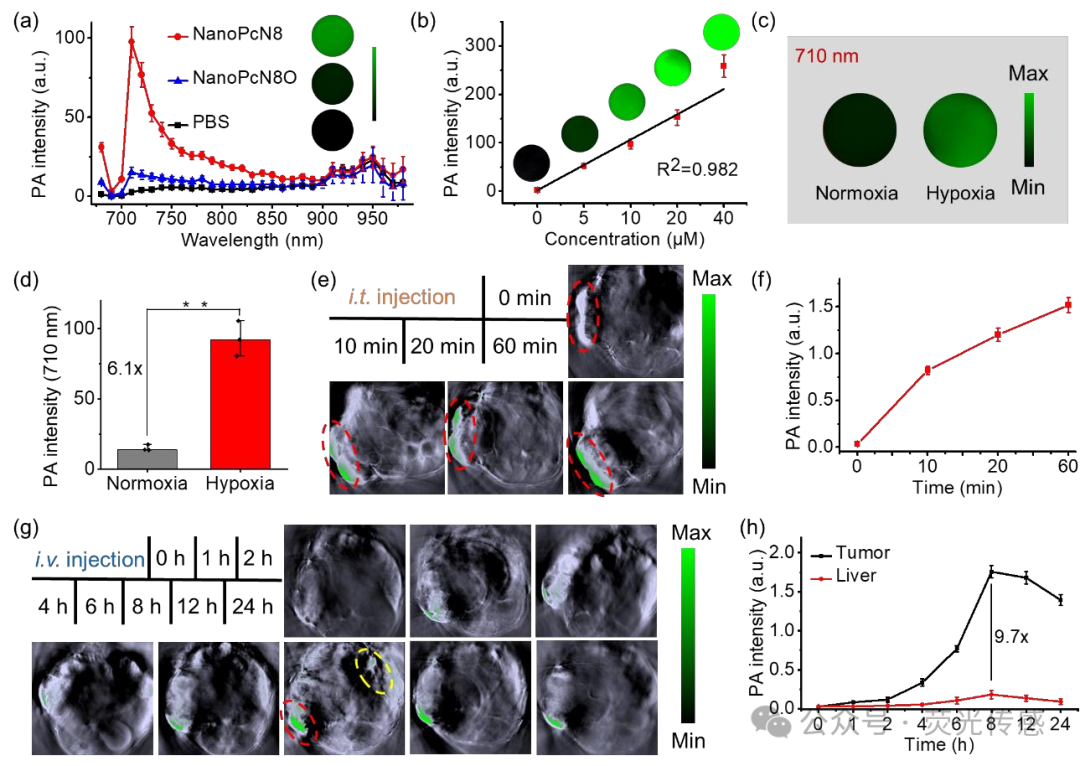
Figure 9. In vivo biodistribution of NanoPcN8O. (a) In vitro PA amplitude of NanoPcN8O and NanoPcN8 (both at 10 μM) at different wavelengths. (b) Linear relationship between PA signal intensity at 710 nm and NanoPcN8 concentration. The insert shows PA images of NanoPcN8 at different concentrations. (c) Representative PA images of NanoPcN8O incubated with NADPH and CYP450 under normoxic or hypoxic conditions for 2 hours (710 nm). (e) Representative PA images of 4T1 tumor-bearing mice before and after intratumoral injection (i.t. injection) of NanoPcN8O (at 690 nm excitation) at different time points. The red ellipse indicates the tumor region. (f) Corresponding quantitative analysis of PA signal intensity at the tumor site in mice after intratumoral injection of NanoPcN8O (dose: 200 μM, 100 μL) at different time points. PA intensity, photoacoustic intensity. (g) Representative PA images of 4T1 tumor-bearing mice before and after intravenous injection (i.v. injection) of NanoPcN8O (at 690 nm excitation) at different time points. The red and yellow ellipses indicate the tumor and liver regions, respectively. (g) Corresponding quantitative analysis of PA signal intensity at the tumor site and liver after intravenous injection of NanoPcN8O (dose: 200 μM, 100 μL) at different time points. PA intensity, photoacoustic intensity.
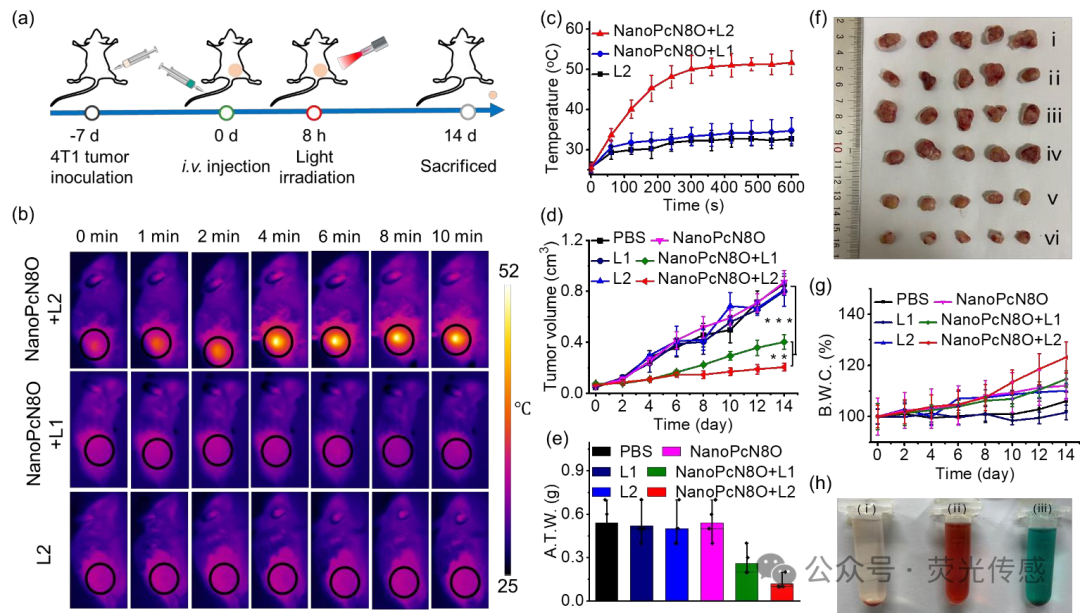
Figure 10. In vivo antitumor effect of NanoPcN8O after intravenous injection (dose: 200 μM, 100 μL) in 4T1 tumor-bearing mice. (a) Schematic diagram showing the treatment of 4T1 tumor-bearing mice with PS. The orange dots represent the tumor. (b) Infrared thermal imaging of 4T1 tumor-bearing mice subjected to specified treatment methods. (c) Temperature change curve of 4T1 tumors after specified treatment. (d) Tumor growth curves of 4T1 tumor-bearing mice after different treatments. R.T.V., relative tumor volume. (e) Average tumor weight and (f) representative tumor images after 14 days of specified treatment. A.T.W., average tumor weight. (i) PBS, (ii) L1, (iii) L2, (iv) NanoPcN8O, (v) NanoPcN8O + L1, and (vi) NanoPcN3O + L2. (g) Average weight change of mice receiving specified treatments. B.W.C., body weight change. (h) Photos of hemolysis phenomena of (i) saline, (ii) water, and (iii) NanoPcN8O (200 μM).
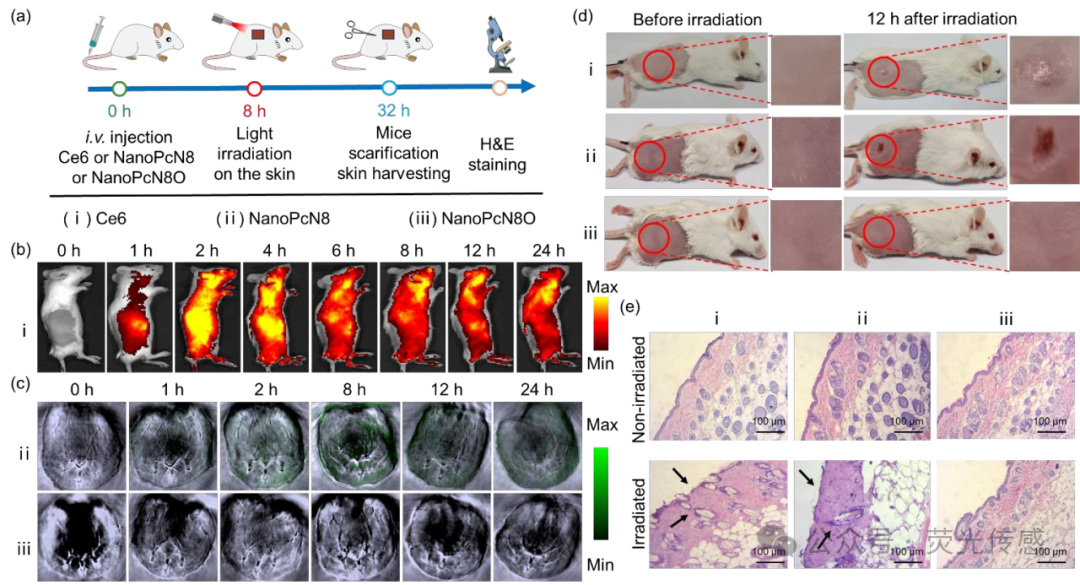
Figure 11. In vivo phototherapeutic effects of NanoPcN8O on mouse skin after intravenous injection. (a) Schematic diagram showing the treatment of mouse skin with PS. (i) Ce6, (ii) NanoPcN8, (iii) NanoPcN8O (all at 200 μM, 100 μL). (b) Fluorescence images of mice before and after intravenous injection of Ce6 aqueous solution containing 1% Cremophor EL at different time points. (c) Representative PA images of mice before and after intravenous injection of NanoPcN8O (at 690 nm excitation) or NanoPcN8 (at 690 nm excitation) at different time points. (d) Photos of mice before and after white light (0.5 W cm-2) irradiation for 10 minutes after injection of NanoPcN8O 8 hours later as a control. (e) H&E staining of mouse skin 24 hours after the above treatments (original magnification 100x).

Summary and Outlook

In conclusion, a hypoxia-induced bioreductive nanotherapeutic agent (NanoPcN8O) has been successfully developed to overcome hypoxia in switchable Type I PDT and PTT tumor microenvironments. Specifically, NanoPcN8O possesses multiple N-oxide groups that can undergo bioreduction via CYP450 enzymes under hypoxic conditions, producing a product NanoPcN8 rich in electron-donating tertiary amine groups, which activates Type I ROS and heat generation. Notably, under hypoxic conditions, NanoPcN8O generated approximately 4.1 times the O2•− levels compared to those induced by MB under the same conditions, enabling NanoPcN8O to overcome tumor hypoxia during PDT. Furthermore, PA imaging indicates that NanoPcN8O selectively activates only in hypoxic tumor tissues in tumor-bearing mouse models. Overall, the Type I photoreaction and photothermal effects enabled by NanoPcN8O significantly inhibited the growth of solid tumors. Better yet, NanoPcN8O does not induce skin phototoxicity, a very common side effect associated with traditional “always-on” PS phototherapy. Therefore, these new findings reported in this study will lead to paradigm shifts in hypoxia imaging and safe phototherapy.
It is noteworthy that NanoPcN8O has high development potential for clinical translation, as PcN8O exhibits significant hydrophilicity. Specifically, PcN8O can be directly dispersed in water, forming a uniform and stable nanosuspension system, with a concentration of up to 29 mg/mL. This makes PcN8O an ideal phototherapeutic agent that can be used directly in biological environments without the aid of organic solvents. Additionally, NanoPcN8O, as an effective phototherapeutic agent, has a tremendous ability to overcome tumor hypoxia. NanoPcN8O exhibits significant phototoxicity under hypoxic conditions, with an IC50 value of 0.14±0.01 μM, which is 86 times lower than that of the commercial PS MB. Importantly, NanoPcN8O can only be activated in hypoxic tumors, generating Type I ROS and heat during phototherapy while remaining non-toxic in normal tissues, thereby minimizing side effects and enhancing the tumor specificity of PDT. Overall, all these unique characteristics favor the clinical translation of NanoPcN8O.
Literature link: https://doi.org/10.1002/anie.202506412
Click “Read the original text” to access the above article