

Pancreatic cancer is a highly malignant and lethal tumor, with a high incidence and extremely poor prognosis. According to the 2021 cancer data from the United States, the incidence of pancreatic cancer continues to rise, ranking 10th among new cases in men and 9th in women, with a mortality rate that ranks 4th among all malignant tumors. The 5-year survival rate is only about 10%, the lowest among all types of cancer, and the mortality rate continues to rise among men.
The only curative treatment for pancreatic cancer is radical resection, but the surgical resection rate for pancreatic cancer patients is less than 20%. Among patients who undergo radical surgical resection, the 5-year survival rate is also less than 40%, and can be as low as 20%. Up to 80% of patients experience local recurrence or distant metastasis after surgery, with most occurring within 2 years post-surgery, and 24% to 51% of patients experiencing recurrence within the first year post-surgery.
To improve patient prognosis, neoadjuvant therapy for pancreatic cancer has received increasing attention. The National Comprehensive Cancer Network (NCCN) in the United States and the 2021 pancreatic cancer diagnosis and treatment guidelines in China recommend neoadjuvant therapy for resectable pancreatic cancer patients with high-risk factors, including significantly elevated CA19-9, large tumor size, suspected metastasis in regional lymph nodes, significant weight loss, and severe pain. However, studies have shown that about 20% of resectable pancreatic cancer patients experience disease progression due to neoadjuvant therapy failure, or miss the opportunity for surgery due to adverse reactions to chemotherapy and decline in physical condition. Preoperative puncture for pathological diagnosis and biliary drainage are also invasive procedures that carry potential risks such as bleeding, cholangitis, pancreatic fistula, and tumor dissemination. Therefore, there is still significant controversy regarding the routine implementation of neoadjuvant therapy for resectable pancreatic cancer patients. There is a need for a reliable preoperative tool to differentiate patients, screening out those at high risk for recurrence and poor prognosis to enter the neoadjuvant therapy process, while non-high-risk patients can proceed directly to radical surgery. Currently, there is no widely recognized screening system accepted by clinical doctors, so this study attempts to analyze the risk factors for early recurrence in patients, thereby establishing a scoring system to classify patients and guide the selection of different treatment strategies for resectable pancreatic cancer patients.

1Materials and Methods
1.1 Study Subjects
Retrospective collection of clinical data from patients with resectable pancreatic cancer who underwent radical surgery in the Department of Hepatobiliary and Pancreatic Surgery, Second Affiliated Hospital of Zhejiang University School of Medicine from March 2015 to June 2021; additionally, patients who underwent radical surgery after neoadjuvant therapy during the same period were included in the neoadjuvant therapy group. Inclusion criteria: (1) underwent radical surgical resection for pancreatic cancer; (2) postoperative pathological diagnosis confirmed as pancreatic ductal adenocarcinoma; (3) preoperative initial imaging assessment indicated resectable pancreatic cancer. Exclusion criteria: (1) did not undergo radical surgical resection; (2) death within 90 days postoperatively; (3) preoperative imaging assessment indicated unresectable; (4) neoadjuvant therapy patients did not obtain pathological evidence preoperatively.
1.2 Observation Indicators
(1) General patient data, including gender, age, body mass index (BMI), history of diabetes, history of hepatitis (hepatitis B/C), smoking and drinking history, preoperative pain, etc. (2) Preoperative imaging data including tumor location, tumor diameter (if different imaging studies measure tumor diameter differently preoperatively, select the data closest to postoperative pathological examination), lymph node status, pancreatic atrophy, pancreatic duct dilation, etc.; preoperative jaundice status, whether preoperative biliary drainage was performed, etc. (3) Preoperative laboratory test indicators (within 2 weeks preoperatively), including complete blood count such as red blood cells, hemoglobin, white blood cells, platelet count, lymphocyte count, etc.; blood biochemistry such as serum albumin, total protein, alanine aminotransferase, aspartate aminotransferase, total bilirubin, glycosylated albumin, total cholesterol, triglycerides, etc.; preoperative coagulation indicators such as international normalized ratio, fibrinogen, etc.; preoperative tumor markers CA19-9, CA242, CA125, CEA, etc. (4) Follow-up data on postoperative recurrence. The median follow-up time was 14 months (1-73 months).
1.3 Relevant Definitions and Standards
(1) The criteria for resectable pancreatic cancer refer to the “Chinese Guidelines for the Diagnosis and Treatment of Pancreatic Cancer (2021)”, where the tumor does not involve the celiac trunk, superior mesenteric artery, or common hepatic artery, and does not involve the superior mesenteric vein or portal vein, or involves but does not exceed 180°, and the vein contour is regular. (2) BMI: body weight (kg)/height2 (m2). (3) Prognostic Nutritional Index (PNI): serum albumin (g/L) + 5 × lymphocyte count (×109/L). (4) GPS score: C-reactive protein (CRP) elevation combined with hypoalbuminemia scores 2, with only one abnormal score 1, both indicators normal score 0. (5) Fibrinogen/platelet count ratio (FPR): fibrinogen (g/mL)/platelet count (×109/L). (6) Early recurrence is defined as recurrence within 6 months postoperatively.

2Results
2.1 Baseline Data
A total of 283 patients with pancreatic cancer who underwent direct radical resection were selected, including 166 males and 117 females, with an average age of (63.77±9.15) years; among them, 95 patients relapsed within 6 months postoperatively, included in the early recurrence group, while the remaining 188 patients without recurrence or with recurrence time exceeding 6 months were included in the non-early recurrence group. During the same period, 20 patients with resectable pancreatic cancer who underwent radical surgery after neoadjuvant therapy were selected, including 11 males and 9 females, with an average age of (59.60±7.61) years; all patients in this group received neoadjuvant chemotherapy, with 3 cases receiving concurrent radiotherapy.
The comparison of baseline data of pancreatic cancer patients who underwent direct radical resection showed that the BMI of the early recurrence group was lower than that of the non-early recurrence group, with a statistically significant difference (P < 0.05). The differences in other baseline indicators were not statistically significant (P values > 0.05) (Table 1).
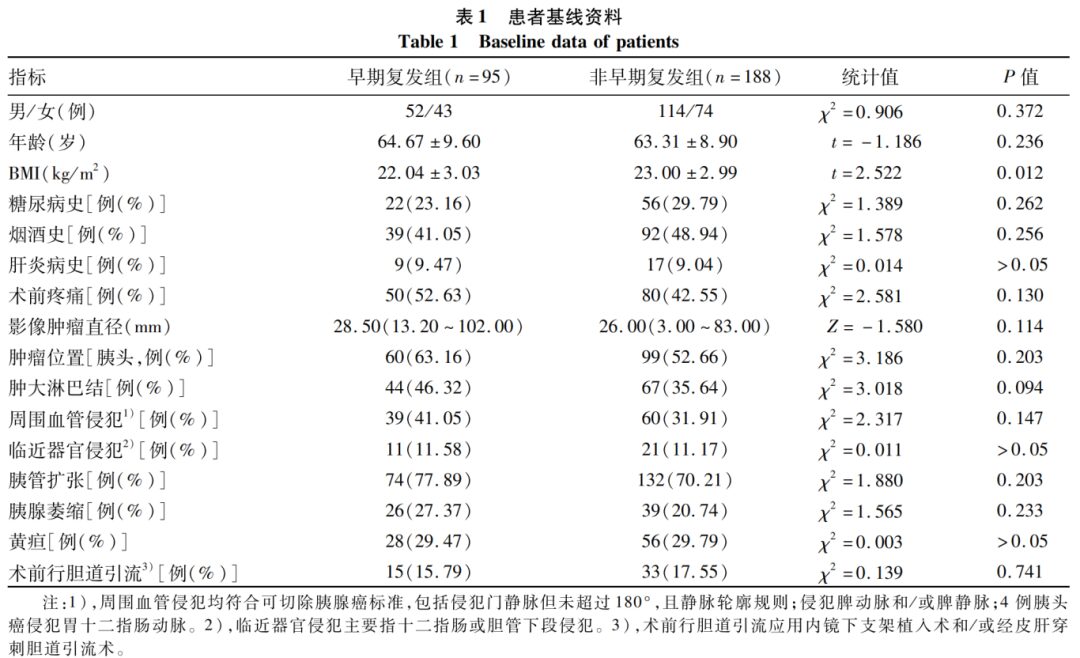
2.2 Comparison of Preoperative Blood Routine Indicators
Comparison of blood routine indicators between the early recurrence group and the non-early recurrence group showed no statistically significant differences in red blood cells, white blood cells, platelet count, etc. (P values > 0.05), as detailed in Table 2.
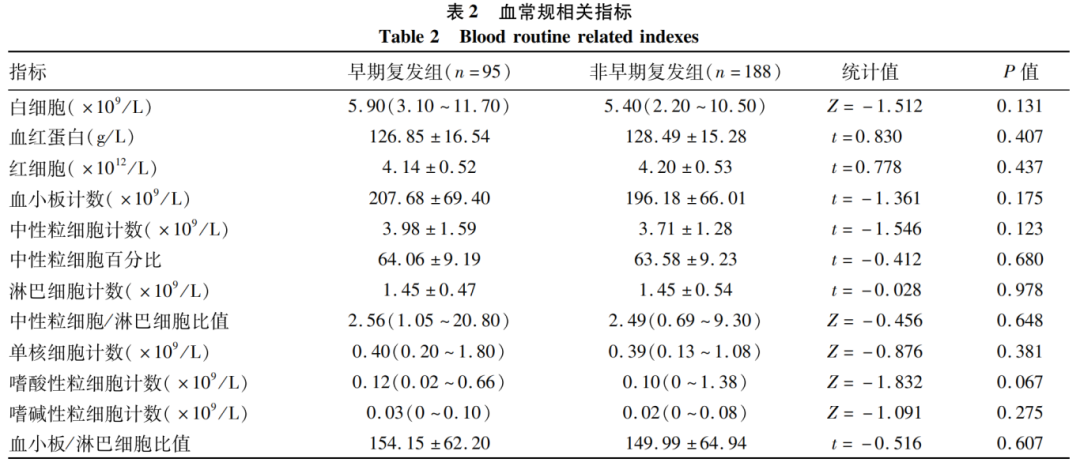
2.3 Comparison of Preoperative Biochemical Indicators
The triglyceride levels in the early recurrence group were lower than those in the non-early recurrence group, with a statistically significant difference (P < 0.05); other biochemical indicators showed no statistically significant differences (P values > 0.05) (Table 3).
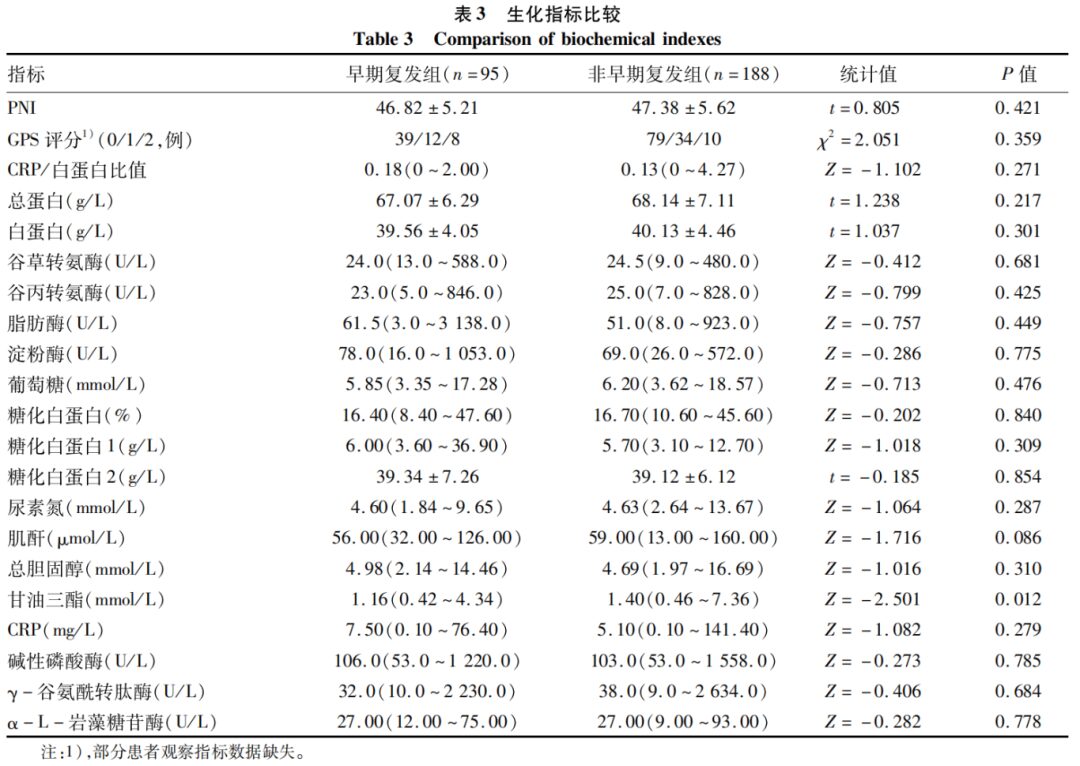
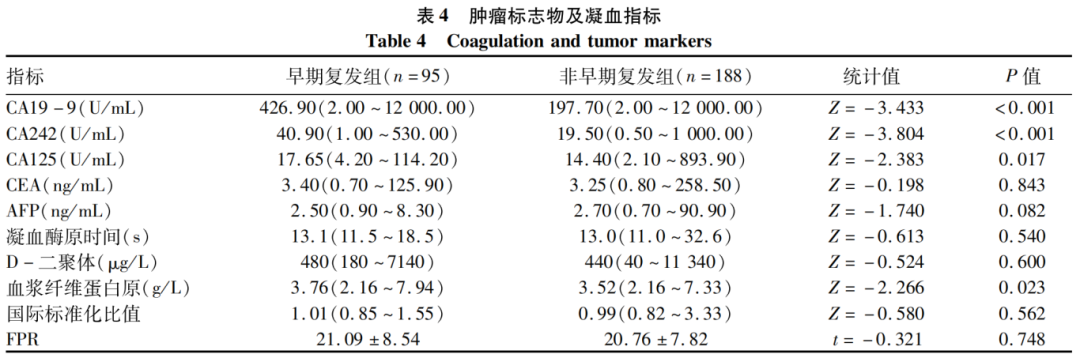
2.4 Comparison of Preoperative Tumor Markers and Coagulation Indicators
The CA19-9, CA242, CA125, and fibrinogen levels in the early recurrence group were higher than those in the non-early recurrence group, with statistically significant differences (P values < 0.05); other tumor markers and coagulation indicators showed no statistically significant differences (P values > 0.05) (Table 4).
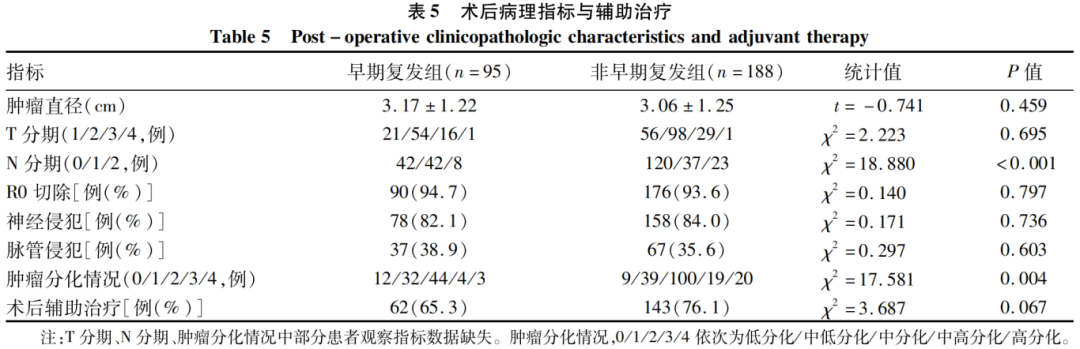
2.5 Comparison of Postoperative Pathological Indicators and Adjuvant Therapy
Compared to the non-early recurrence group, the N stage (lymph node positivity rate) and tumor differentiation status in the early recurrence group showed statistically significant differences (P values < 0.05), while other postoperative pathological indicators and adjuvant therapy showed no statistically significant differences between the two groups (P values > 0.05).
2.6 Establishment and Validation of Scoring System
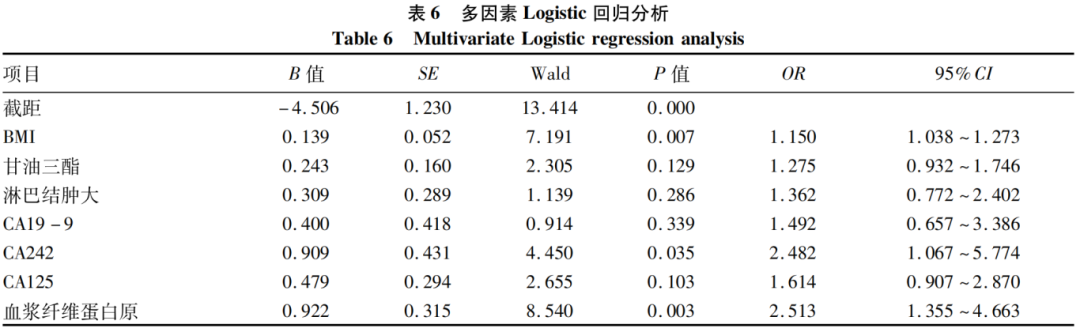
The best cut-off values of statistically significant indicators from univariate analysis were determined through ROC curves, which were converted into binary variables for further multivariate analysis. The results showed that the best cut-off values for BMI, CA19-9, CA242, CA125, and fibrinogen were 23.00 kg/m2, 260.0 U/mL, 30.0 U/mL, 15.0 U/mL, and 4.00 g/L, respectively. The cut-off values were converted into binary variables for scoring, where imaging suggesting enlarged lymph nodes = 1, non-enlarged lymph nodes = 0; BMI < 23.00 kg/m2 = 0, ≥23.00 kg/m2 = 1; CA19-9 < 260.0 U/mL = 0, ≥260.0 U/mL = 1; CA242 < 30.0 U/mL = 0, ≥30.0 U/mL = 1; CA125 < 15.0 U/mL = 0, ≥15.0 U/mL = 1; fibrinogen < 4.00 g/L = 0, ≥4.00 g/L = 1. The variables of imaging suggesting enlarged lymph nodes, CA19-9, CA242, CA125, fibrinogen, BMI, and triglycerides were included in the multivariate logistic regression analysis, which showed that BMI, fibrinogen, and CA242 were independent risk factors for early recurrence in resectable pancreatic cancer patients (P values < 0.05) (Table 6).
The scoring system included three indicators: BMI, CA242, and fibrinogen. A score of 1 was assigned for BMI < 23.00 kg/m2, CA242 ≥ 30.00 U/mL, and fibrinogen ≥ 4.00 g/L, while a score of 0 was assigned otherwise, resulting in a total score of 0 to 3. When scoring early recurrence and non-early recurrence patients, the results showed that the early recurrence group had a higher score [2(0-3) vs 1(0-3), Z=-5.339, P < 0.001]. Kaplan-Meier curve analysis showed that there was a statistically significant difference in recurrence-free survival time among different scoring groups (χ2=28.116, P < 0.001) (Figure 1), with higher scores indicating shorter expected recurrence-free survival time. The median recurrence-free survival times for the four groups were 29 months, 12 months, 7 months, and 3 months, with an overall median recurrence-free survival time of 11 months. A score of 3 was defined as the high-risk group, while 0-2 points were defined as the low-risk group. Among non-neoadjuvant therapy patients, the early recurrence rate in the high-risk group was 84.6%, while in the low-risk group it was 31.2%. In the neoadjuvant therapy group, there were 3 high-risk patients, with recurrence-free survival times of 9 months, 12 months, and 60 months, showing no statistically significant difference compared to high-risk patients in the non-neoadjuvant therapy group (P=0.153); there was also no statistically significant difference in recurrence-free survival time between low-risk patients receiving neoadjuvant therapy and those receiving non-neoadjuvant therapy (P=0.755). The pathological indicators of the high-risk and low-risk groups showed no statistically significant differences (P values > 0.05).
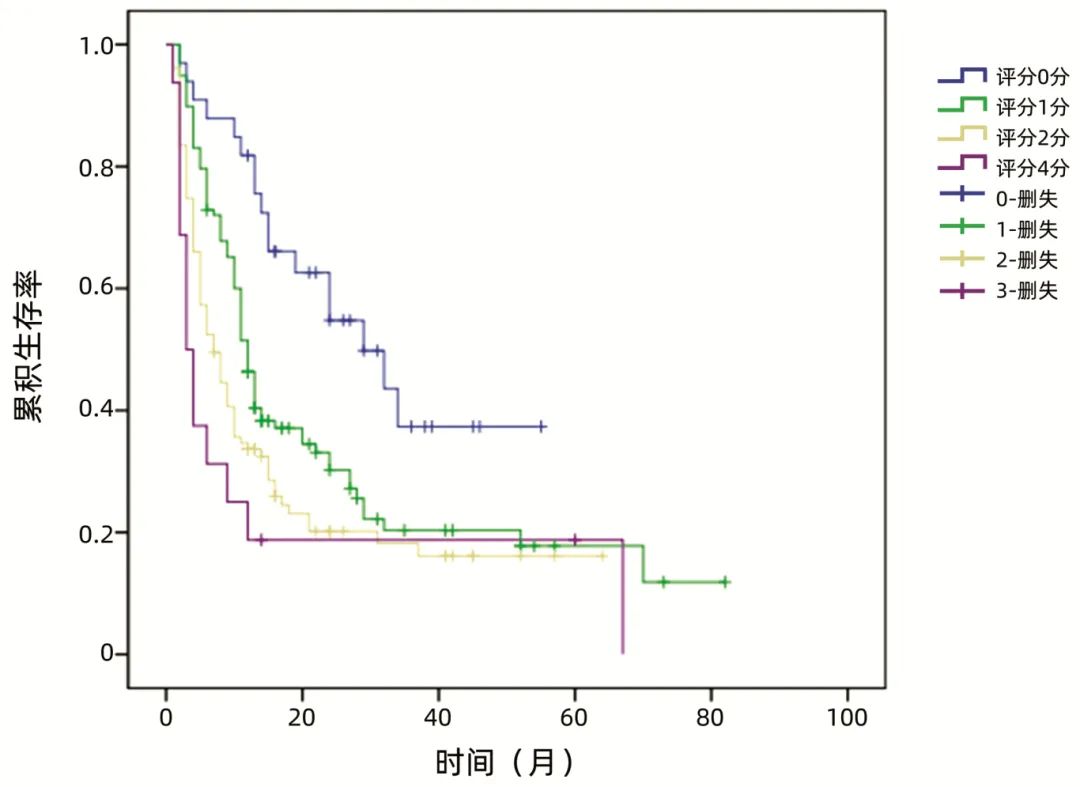
Figure 1: Recurrence-free survival curves for patients with different scores

3Discussion
After radical resection of pancreatic cancer, the R0 resection rate is only 70%-76%. Studies have shown that most pancreatic cancer patients, even those with very small localized lesions, may already have micrometastases at the time of diagnosis. Neoadjuvant therapy can improve the R0 resection rate of pancreatic cancer and reduce lymph node metastasis, thereby improving patient prognosis. Current domestic and international guidelines recommend that patients with locally borderline resectable pancreatic cancer and locally advanced pancreatic cancer undergo preoperative neoadjuvant or conversion therapy to achieve better prognosis.
For patients with resectable pancreatic cancer, there is still significant controversy regarding whether to implement neoadjuvant therapy. On one hand, there is currently no high-quality evidence indicating that neoadjuvant therapy can improve the prognosis of patients with resectable pancreatic cancer; on the other hand, patients face the risk of treatment failure, tumor progression, and loss of surgical opportunity. In the study by Kurahara et al., patients with resectable pancreatic cancer were grouped based on CA19-9 and P53 expression, showing that neoadjuvant therapy significantly improved overall survival in the high-risk group, while there was no difference in survival between neoadjuvant therapy and direct surgery in the low-risk group, indicating the necessity and clinical value of preoperative classification for resectable pancreatic cancer. Current guidelines for high-risk factors in resectable pancreatic cancer are relatively vague, limiting their practical application value. This study aims to identify high-risk factors for early recurrence in resectable pancreatic cancer, and thus obtain a preoperative grading method to guide individualized treatment for patients.
Currently, there is no unified concept regarding early recurrence after radical surgery for pancreatic cancer. Previous literature has defined early recurrence as recurrence within 12 months or 6 months postoperatively. In this study, 52.7% of patients relapsed within 12 months, and 33.6% relapsed within 6 months post-surgery, with a median recurrence time of 11 months. Considering the data from this study and referencing literature, early recurrence is defined as recurrence within 6 months postoperatively.
Guidelines indicate that high-risk factors for resectable pancreatic cancer include large tumor size, suspected metastatic lymph node enlargement, significant weight loss, and severe pain. Research by Hank et al. showed that age > 70 years and male gender were associated with shorter survival in univariate analysis, but did not show significant differences in multivariate analysis. The results of this study showed that BMI was lower in early recurrence patients, similar to the guideline’s indication of significant weight loss, reflecting the nutritional status of patients and demonstrating its predictive value in prognosis.
Some scholars believe that a preoperative tumor diameter > 3 cm is a risk factor for early recurrence. Research by Kim et al. indicated that preoperative CT tumor diameter, low-density lesions in the portal vein phase, tumor necrosis, peritumoral infiltration, and suspected lymph node metastasis are risk factors for postoperative recurrence and death. However, this study did not show predictive value for early recurrence based on preoperative imaging examination data. There were no statistically significant differences in tumor diameter, surrounding lymph node enlargement, adjacent vascular invasion, pancreatic duct dilation, or pancreatic atrophy between the early recurrence group and the non-early recurrence group, suggesting limitations of simple imaging assessment. Moreover, regardless of whether the tumor diameter was measured by preoperative imaging or postoperative pathology, the smallest tumor in the early recurrence group was only about 1 cm, and there was no significant difference in tumor diameter between the early recurrence and non-early recurrence groups, indicating that even very small pancreatic cancers may experience early recurrence and metastasis.
Blood routine and related indicators can reflect inflammatory and nutritional status in patients and are used to assess the prognosis of pancreatic cancer patients, including neutrophil/lymphocyte ratio, platelet/lymphocyte ratio, platelet count, etc. Okabayashi et al. included 240 patients with resectable pancreatic cancer and found that the neutrophil/lymphocyte ratio is an independent predictive factor for survival after radical surgery, with patients having a neutrophil/lymphocyte ratio > 2.5 experiencing shorter recurrence times and survival. Research by Hank et al. indicated that platelet count is a predictive factor for survival after pancreatic cancer surgery, with patients having a platelet count < 150/nL showing shorter survival. Another study suggested that platelet count/lymphocyte ratio is an effective prognostic marker after pancreatic cancer radical surgery. This study also analyzed preoperative blood routine indicators, but no statistically significant differences were found between the two groups, indicating no predictive value.
Some studies have shown that GPS scores and CRP/albumin ratios can predict pancreatic cancer prognosis. Other studies have demonstrated that patients with low PNI experience earlier recurrence and poorer postoperative survival. Research by Hank et al. showed that patients with CRP ≥ 20 mg/L and albumin < 35 g/L had significantly shorter survival. These studies indicate the value of inflammatory and nutrition-related indicators in predicting pancreatic cancer prognosis. The occurrence of malignant tumors is related to chronic aseptic inflammatory responses, which trigger inflammatory reactions that further accelerate the malignant process. Tumor-associated inflammatory responses recruit regulatory T lymphocytes and activate chemokines, thereby inhibiting anti-tumor immunity to promote tumor growth and metastasis. In this study, except for relatively low triglyceride levels in early recurrence patients, no differences were observed in other indicators, thus the value of these indicators remains to be further verified by research.
CA19-9 is an important tumor marker for pancreatic cancer. In addition, some studies have shown that CA125 has prognostic assessment value in CA19-9 negative pancreatic cancer patients, and preoperative CEA and CA125 levels above normal with CA19-9 ≥ 1000 U/mL can be considered high-risk factors for poor prognosis. Chinese guidelines recommend the combined use of CA19-9, CEA, CA125, and CA242 for diagnosing pancreatic cancer. Previous studies have shown inconsistent predictive value for CA19-9 prognosis, with varying cut-off values. Research by Hank et al. indicated that patients with CA19-9 ≥ 400 U/mL and CEA ≥ 2.5 μg/L have poorer prognosis. In the study by Okabayashi et al., patients with CA19-9 ≥ 300 U/mL had worse prognosis and were more likely to experience early recurrence. The study by Kurahara et al. indicated that CA19-9 > 85 U/mL suggests a higher likelihood of early recurrence (within 6 months postoperatively). In contrast, research by Groot et al. set the cut-off value for CA19-9 at > 210 U/mL. This study’s results showed that early recurrence patients had higher levels of CA19-9, CA242, and CA125, with cut-off values of 260.0 U/mL, 30.0 U/mL, and 15.0 U/mL, respectively. However, multivariate logistic regression analysis indicated that only CA242 was an independent risk factor for early recurrence.
Coagulation indicators are also closely related to pancreatic cancer. Pancreatic cancer is associated with the highest risk of venous thromboembolism, and thromboembolic events are predictive factors for early (within 3 months) death in pancreatic cancer patients. Additionally, D-dimer can be found in resectable pancreatic cancer without thrombosis and is associated with poor prognosis. These studies indicate that hypercoagulable states, microthrombosis, and eventual thrombosis are pathological states in the development of pancreatic cancer, closely related to prognosis. The author included coagulation-related indicators in the study and found that high fibrinogen levels significantly shortened recurrence time. Research by Arakawa et al. showed that patients with FPR < 25.2 had significantly improved overall survival and recurrence-free survival. However, this study did not find an impact of FPR and other coagulation indicators on prognosis.
Through multivariate logistic regression analysis, BMI, CA242, and fibrinogen were included in the scoring system, indicating that this scoring system has good predictive effects in this study. Compared to patients receiving neoadjuvant therapy, neither the high-risk nor low-risk groups showed significant recurrence-free survival benefits (P > 0.05), while the high-risk group showed a trend towards improved recurrence-free survival time, suggesting that this scoring system has certain clinical value. Previous literature has also established prognostic scoring systems for resectable pancreatic cancer based on their respective predictive factors, achieving good predictive effects. However, most studies, like this one, are based on single-center data analysis, leading to significant differences in inclusion indicators and final results, which is also the reason why no scoring system has been widely recognized and applied.
Previous studies have indicated that TNM staging, lymph node positivity, and nerve vascular invasion are risk factors for recurrence and death after pancreatic cancer surgery. In this study, the early recurrence group had a higher lymph node positivity rate and more poorly differentiated patients, but the analysis results did not show correlations between preoperative risk factors and the scoring system with various pathological indicators. The early recurrence risk factors in this study may not affect the pathological indicators of pancreatic cancer but rather impact prognosis through other factors such as inflammatory factors, immune status, postoperative adjuvant chemotherapy, circulating tumor cells, and biological behavior.
Studies by Strijker et al. have shown that serum ADAM12 levels have predictive value for survival in pancreatic cancer patients; Kurahara et al. found that P53 expression in pancreatic cancer cells can predict early postoperative recurrence; and research by Dreyer et al. demonstrated that high expression of calcium-binding proteins S100A2 and S100A4 in pancreatic cancer tissues indicates poor prognosis. These biomarkers may be applied for preoperative prediction of pancreatic cancer prognosis, although further validation with larger sample sizes is needed, they provide direction for future research.
The limitations of this study are as follows: first, it is a single-center study with a relatively small sample size, especially for patients receiving neoadjuvant therapy, which may introduce bias and affect the efficacy and promotion of this scoring system. Second, there is a lack of sufficient validation sets for verification. Validating the scoring system on the original cases may lead to an overestimation of result conformity.
In summary, BMI, CA242, and fibrinogen are independent risk factors for early recurrence in resectable pancreatic cancer patients. These three indicators are included in the scoring system, where BMI < 23.00 kg/m2, CA242 ≥ 30.00 U/mL, and fibrinogen ≥ 4.00 g/L are each assigned 1 point, while others are assigned 0 points, yielding a total score of 0 to 3. The aforementioned risk factors and scoring system are independent of postoperative pathological factors and TNM staging, and can predict postoperative recurrence to some extent. A score of 3 indicates a high-risk group with a high risk of early recurrence, which may benefit from preoperative neoadjuvant therapy; a score of 0-2 indicates a low-risk group that may proceed directly to radical surgical treatment or receive treatment plans after multidisciplinary consultation.

https://www.lcgdbzz.org/cn/article/doi/10.3969/j.issn.1001-5256.2022.10.023

Wang Chengfang, Wang Zhijiang, Wang Weilin. Establishment and Application of Preoperative Scoring System for Resectable Pancreatic Cancer[J]. Clinical Journal of Hepatobiliary Diseases, 2022, 38(10): 2325-2333


Thematic Issues and Executive Editors of Clinical Journal of Hepatobiliary Diseases 2024 Issue 1-11

Expert Forum | Chen Yuanwen: Basic Research on Chronic HBV Infection and Metabolic Dysfunction – Current Progress and Controversies

Expert Forum | Zheng Qi: Epidemiology and Clinical Outcomes of Chronic HBV Infection and Metabolic Dysfunction