

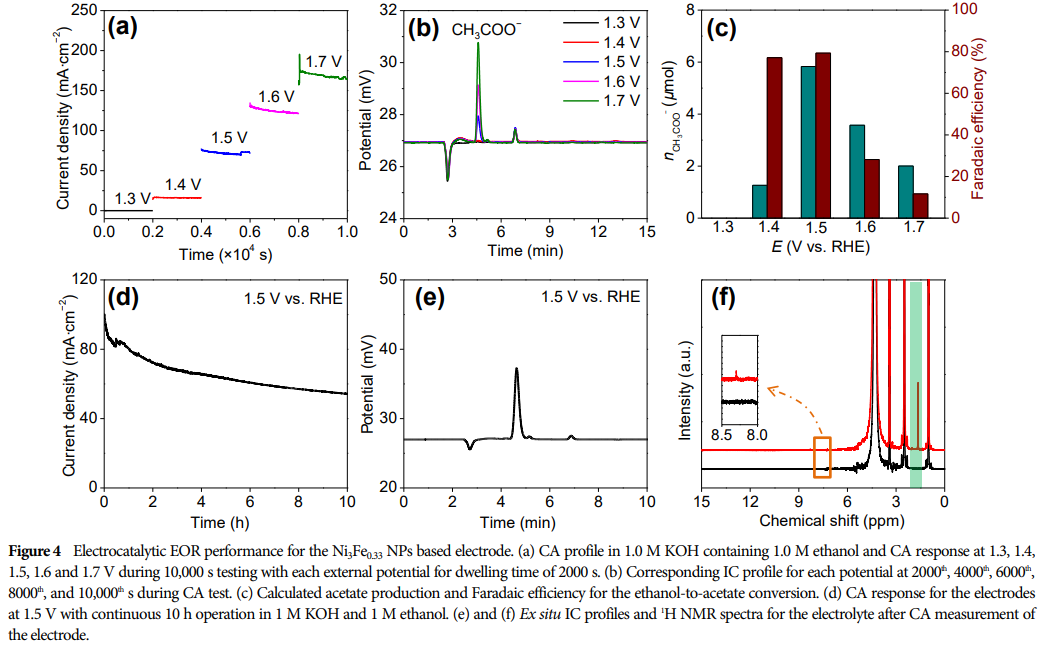

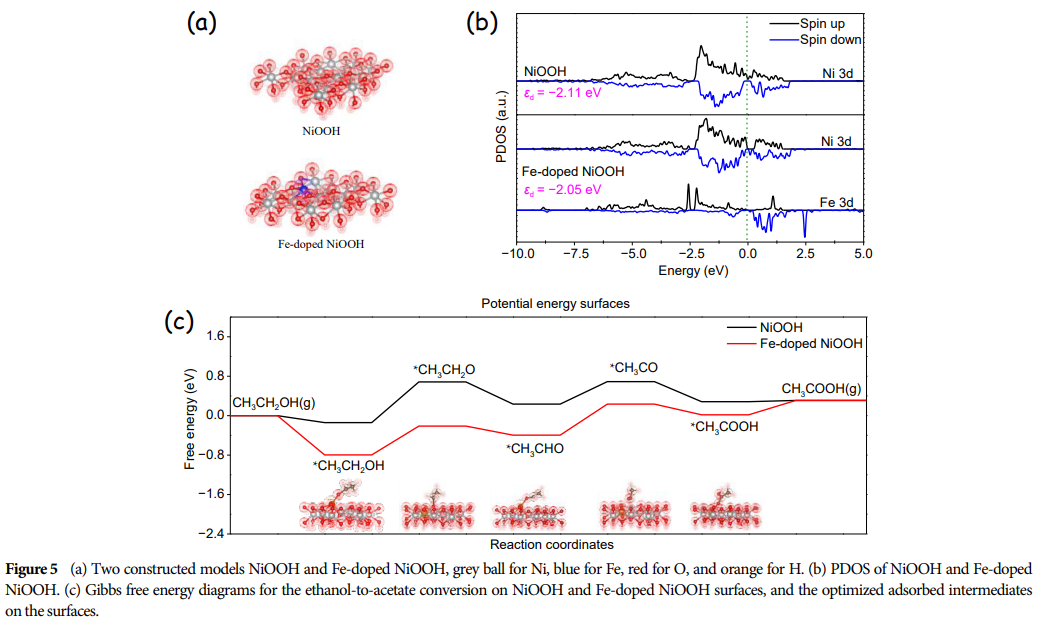
-
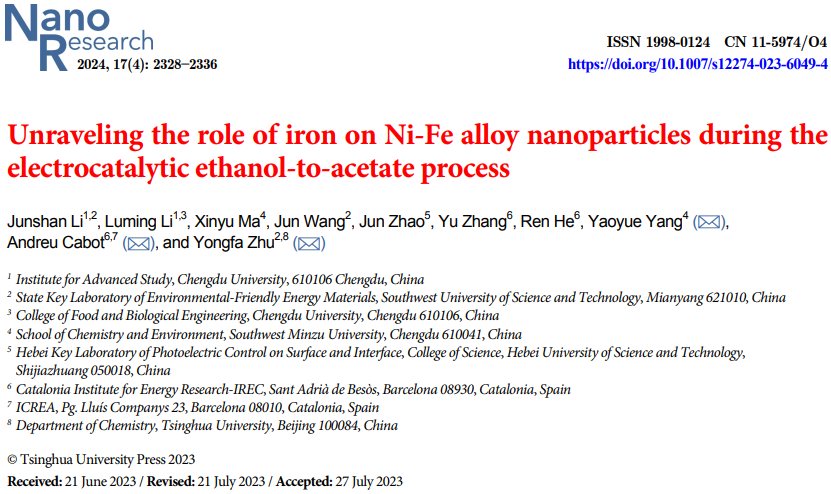
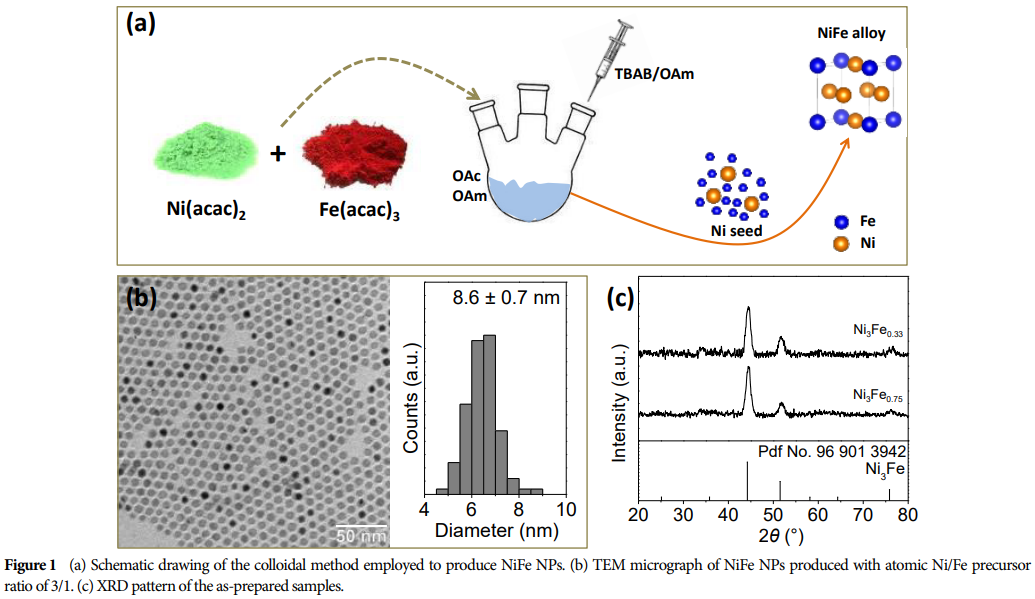
This paper synthesized Ni-Fe alloy nanoparticles through a colloidal strategy, serving as efficient electrocatalysts for ethanol electrooxidation in alkaline media. -
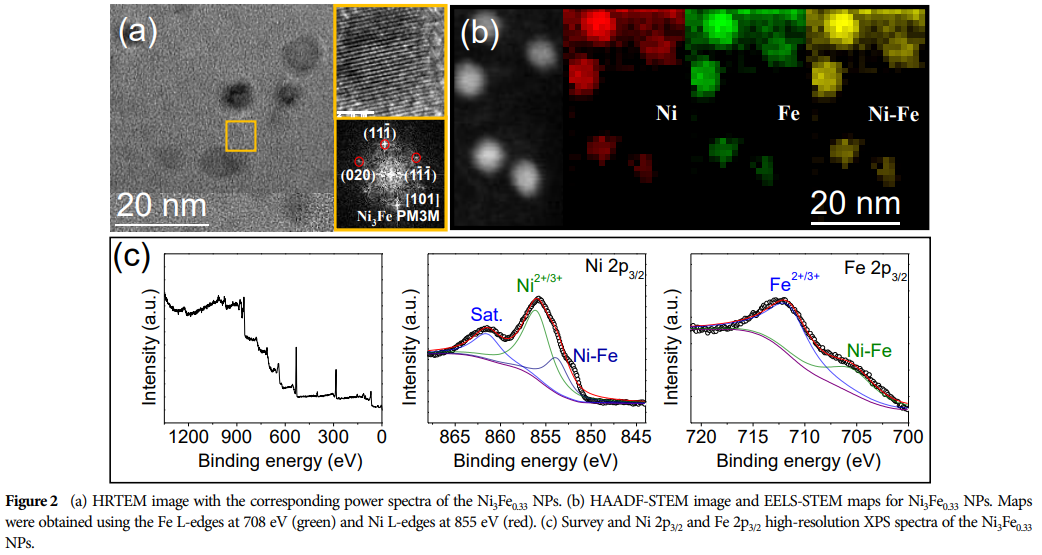
Ni-Fe alloy NPs exhibit excellent electrochemical performance, achieving high current densities at low potentials and producing high yields of acetic acid. -
DFT calculations reveal the influence of iron doping on the mechanism of ethanol electrooxidation, providing theoretical guidance for designing more efficient electrocatalysts. -
Moreover, this study offers new insights for replacing the oxygen evolution reaction to promote the cathodic hydrogen evolution reaction and save energy.
