

The hand is a very important organ, not only for humans but also for many mammals that use their hands to interact with the environment. We can accurately aim at targets with our hands, such as hitting a rapidly moving ping-pong ball by controlling hand movements; we can also skillfully manipulate objects, such as playing the piano with our fingers. Thus, aiming and manipulation are the two main functions of the upper limbs (including the hands), and to achieve these functions, we must control the contraction of upper limb muscles through specific neural circuits composed of particular nerve cells.
Existing research indicates that motor-related neural circuits are distributed across multiple regions from the brain to the spinal cord【1】. Some of these neural circuits are located in the hindbrain, including the brainstem and spinal cord, responsible for sending neural signals to control muscle activity to execute motor functions; others are located in the cerebral cortex and basal ganglia, responsible for integrating various information related to motor sensations and sending signals to control downstream neural circuits【2】. Recent studies have identified that neurons in specific regions of the medulla in the brainstem can act like a switchboard, selecting different medullary/spinal cord neural circuits to control various upper limb motor functions【3】. So, how do these medullary neurons related to upper limb movement emit signals at specific times in appropriate ways to accurately guide the upper limbs in executing motor functions?
The cerebral cortex, due to its extensive neural pathways projecting to the medulla, can influence and regulate neural circuits in multiple brain regions to complete the processing of motor signals. Therefore, understanding the connection logic between motor neural circuits in different regions of the nervous system, especially how neural signals from the cerebral cortex are transmitted to the medulla, is crucial for further exploring the neural basis of upper limb motor functions, studying the mechanisms of motor learning, and even promoting the recovery of motor functions after injury.
On January 5, 2023, the research group of Silvia Arber from the Biozentrum of the University of Basel and the Friedrich Miescher Institute (with Yang Wuzhou as the first author) published an article titled Structural and functional map for forelimb movement phases between cortex and medulla in Cell, which anatomically analyzes the structure of the neural pathways from the cerebral cortex to the medulla and reveals the important role of specific cortical-medullary neural projections in controlling the aiming and manipulation functions of upper limb movements.


Welcome to join
the NationalSports and Brain ScienceAcademic Discussion Group
the NationalNeural CircuitAcademic Discussion Group
Add the editor’s WeChat
brainnews_09
-Message: Sports and Brain Science, Neural Circuit research group-


First, to determine which neurons in the cerebral cortex can directly communicate with the medullary neural circuits related to upper limb movement, the authors used fluorescently labeled retrograde adeno-associated virus (AAV)【4】. This type of virus can infect neurons through axons, thus marking neurons projecting to specific areas. The authors injected this AAV into the medullary region related to upper limb movement and found that cortical-medullary projection neurons are mainly distributed in the anterior part of the cerebral cortex. By comparing with the distribution of cortical-spinal projection neurons, the authors found that in the anterior cerebral cortex, the medial area (MAC) has both cortical-medullary and cortical-spinal projection neurons, while the lateral area (LAC) only has cortical-medullary projection neurons.
Thus, the authors identified that different regions of the cerebral cortex have different projection neurons, and how do these cortical regions communicate with the medulla? To study whether different regions of the cerebral cortex have specific cortical-medullary neural projections, the authors used anterograde tracing AAV to label the synapses of cortical neurons within specific regions.
Interestingly, the authors found that MAC projects to the ventral medulla, while LAC projects to the dorsal medulla, and furthermore, this topological projection remains consistent throughout the medulla from anterior to posterior, thus forming a three-dimensional columnar structure of synapses from the cerebral cortex to the medulla. This anatomical structure suggests specific cortical-medullary neural pathways and demonstrates the fine subdivision of the cerebral cortex.
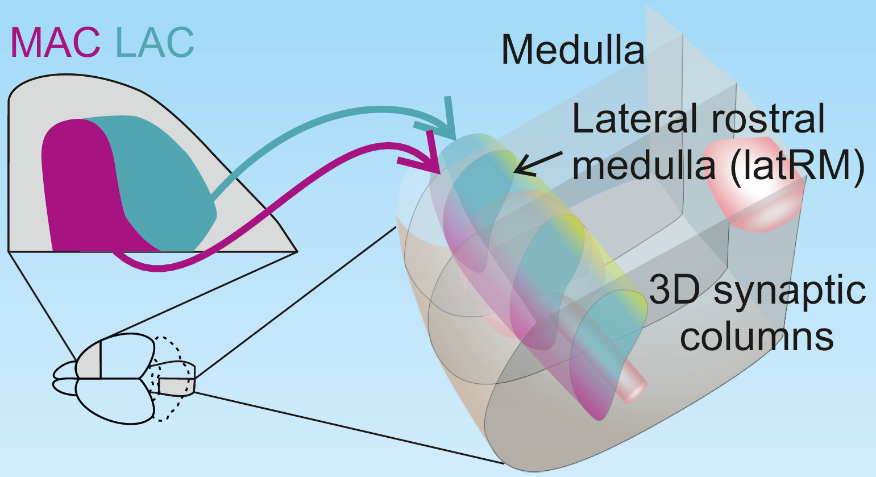
Figure 1: Three-dimensional structure of cortical to medullary neural projections
So, does this fine subdivision of the cerebral cortex have functional significance? To determine the roles of different cortical regions in upper limb movement, the authors trained mice to reach out and grab food pellets. Interestingly, when MAC was temporarily inactivated, the mice lost the ability to reach out, while the temporary inactivation of LAC had no effect on the reaching movement compared to the control group.
On the other hand, when the mice grabbed and ate the food pellets, they used both hands to manipulate the food. Surprisingly, compared to the temporary inactivation of MAC and the control group, the temporary inactivation of LAC made the mice less likely to use both hands to manipulate the food pellets, and even when the mice used both hands, those with temporary inactivation of LAC still could not manipulate the food pellets well. Thus, the authors concluded that MAC and LAC play necessary roles in controlling the aiming and manipulation of upper limb movements, respectively.
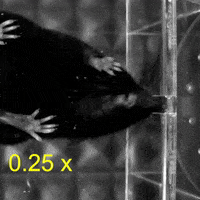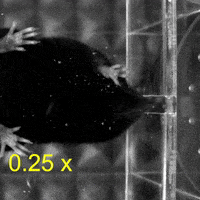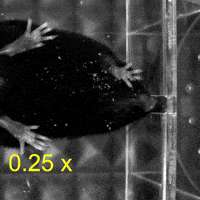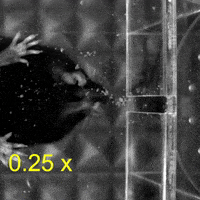
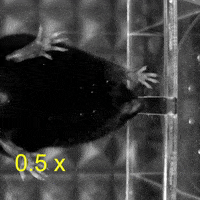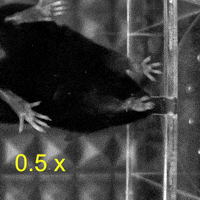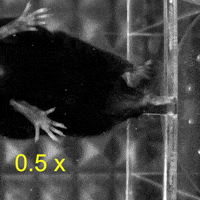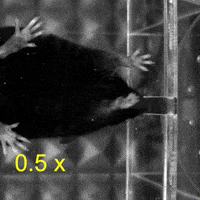
Video 1: MAC plays a necessary role in reaching and aiming: Top: Control; Bottom: MAC inactivated.
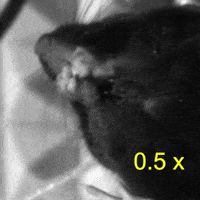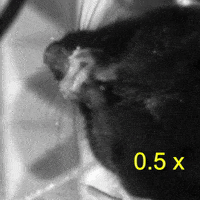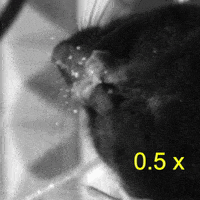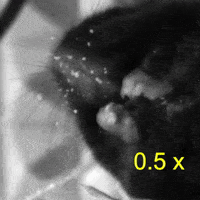
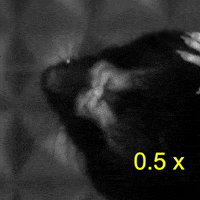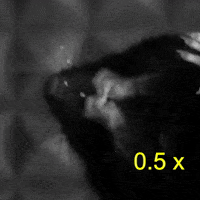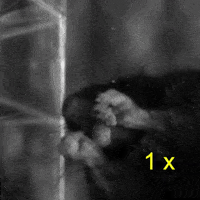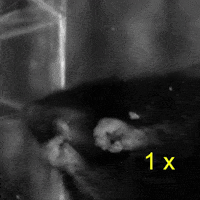
Video 2: LAC plays a necessary role in two-handed manipulation. Top: Control; Bottom: LAC inactivated.
The further question becomes, is the specific function of the cerebral cortex related to the neural circuits in the medulla? To answer this question, the authors first used trans-synaptic labeling AAV to observe the postsynaptic neurons in the medulla that receive projections from the cerebral cortex【5】. The results showed that these postsynaptic neurons in the medulla have a similar synaptic distribution to those from the cerebral cortex, suggesting that different medullary neurons receive specific signals from the cerebral cortex.
Furthermore, the authors recorded the discharge activity of upper limb movement-related medullary neurons in freely moving mice using in vivo multi-channel silicon electrodes and utilized optogenetics to express light-sensitive ion channels to stimulate different cortical regions, thereby recording the activity of medullary neurons regulated by the cortex. While recording neuronal activity, the mice also performed a series of movements to reach out and grab food pellets and manipulate the food pellets with both hands, allowing the activity of medullary neurons to be associated with the upper limb movement behavior of the mice.
Interestingly, the medullary neurons regulated by MAC were mostly related to the mice reaching for food, while the medullary neurons regulated by LAC exhibited discharge activity when the mice manipulated the food pellets. This experimental result indicates that different regions of the cerebral cortex transmit neural signals to specific medullary neurons, and these specific medullary neurons participate in upper limb movements at different phases, from aiming to manipulation.
In summary, this study reveals the fine subdivision of the cerebral cortex and discovers specific neural connection architectures between the cerebral cortex and the medulla, which accurately regulate a series of upper limb movements from aiming to manipulation. The authors’ further research indicates that this highly specialized cortical neural projection pathway exists not only in the medulla but can also extend to more motor-related areas, providing a foundation for further research on the neural control mechanisms of upper limb movement.
Original link:
https://doi.org/10.1016/j.cell.2022.12.009
References
1. Lemon, R.N. (2008). Descending Pathways in Motor Control. Annu. Rev. Neurosci. 31, 195–218. 10.1146/annurev.neuro.31.060407.125547.
2. Arber, S., and Costa, R.M. (2022). Networking brainstem and basal ganglia circuits for movement. Nat. Rev. Neurosci., 1–19. 10.1038/s41583-022-00581-w.
3. Ruder, L., Schina, R., Kanodia, H., Valencia-Garcia, S., Pivetta, C., and Arber, S. (2021). A functional map for diverse forelimb actions within brainstem circuitry. Nature 590, 445–450. 10.1038/s41586-020-03080-z.
4. Tervo, D.G.R., Hwang, B.-Y., Viswanathan, S., Gaj, T., Lavzin, M., Ritola, K.D., Lindo, S., Michael, S., Kuleshova, E., Ojala, D., et al. (2016). A Designer AAV Variant Permits Efficient Retrograde Access to Projection Neurons. Neuron 92, 372–382. 10.1016/j.neuron.2016.09.021.
5. Zingg, B., Chou, X., Zhang, Z., Mesik, L., Liang, F., Tao, H.W., and Zhang, L.I. (2017). AAV-Mediated Anterograde Transsynaptic Tagging: Mapping Corticocollicular Input-Defined Neural Pathways for Defense Behaviors. Neuron 93, 33–47. 10.1016/j.neuron.2016.11.045.
