


Research Background
To break the limitations of mechanism research and material design, it is necessary to have a comprehensive understanding of lithium-ion dynamics in batteries. Kinetic processes with specific relaxation characteristics can be identified on a timescale. Extracting and analyzing timescale information in batteries will provide profound insights into kinetic issues such as ion conduction, charge transfer, diffusion, interface evolution, and other unknown kinetic processes. In this regard, timescale identification can be combined with non-destructive impedance characterization on a length scale for online battery monitoring. This method introduces timescale characterization, utilizing the concept of Distribution of Relaxation Times (DRT), and has achieved successful applications in battery diagnostics. In the future, timescale characterization will become a powerful data tool for extracting and constructing datasets for various battery systems.

Summary of Results
Professor Zhang Qiang from Tsinghua University and Postdoctoral Zhao Chenzi (co-corresponding authors) and others introduced the fundamentals, specifications, applications, and prospects of timescale analysis for various battery systems, such as solid-state batteries, metal-S/O2 batteries, and metal-ion batteries. This review was published under the title The Timescale Identification Decoupling Complicated Kinetic Processes in Lithium Batteries in the journal Joule.

Content Details
The timescale effects in batteries mainly arise from four physical processes: electric double layer, local charge concentration, charge balance, and concentration gradients in the electrolyte or electrode materials. External stimuli (such as current or voltage) can induce relaxation processes under different conditions. Relaxation refers to the recovery process after external disturbance, which is an intrinsic property of isolated systems. Therefore, different relaxation properties can be used to distinguish kinetic processes: conduction, adsorption, and release of lithium ions at the interface. This allows for quantitative analysis of the battery system’s kinetics, which is also an extension of traditional EIS testing.
The relationship between Nyquist plots, DRT plots, and ECM is shown in Figure 1. Ideally, a typical EIS consists of separated semicircles. Each semicircle is associated with a specific time constant, which appears as isolated lines in the DRT plot, corresponding to their respective parallel resistance and capacitance circuits. In practice, the semicircles in the EIS plot are coupled, making it difficult to distinguish them. DRT can transform coupled EIS into a continuous curve with several specific peaks, representing constant phase elements (CPE), affecting parallel capacitance and resistance. The relevant ECM model can be obtained based on the deconvolution time constants.
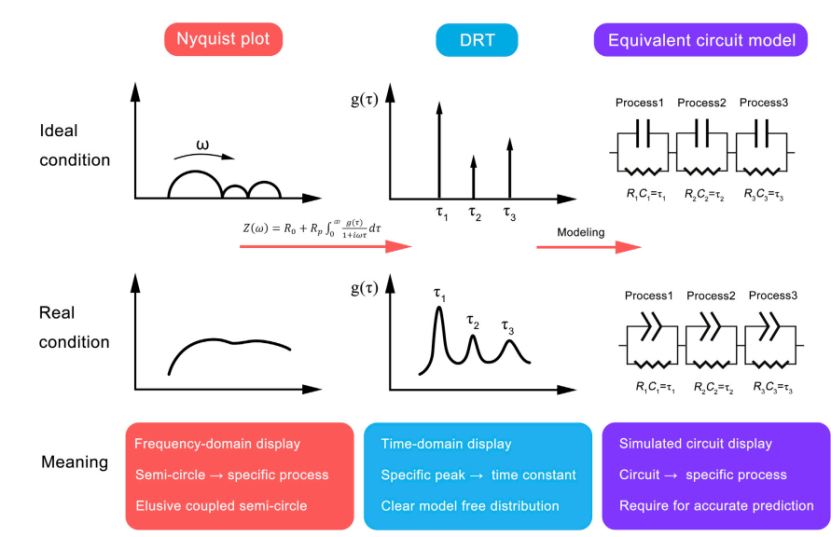
Figure 1 Relationship between Nyquist plot, ideal equivalent circuit model, and real condition electrochemical model.
Typical Procedure for Battery DRT Diagnosis
Distributing the time constants of electrochemical processes is the focus of EIS research. Determining their timescales is fundamental to interpreting EIS. Figure 2 shows a typical workflow for DRT diagnosis in battery research.
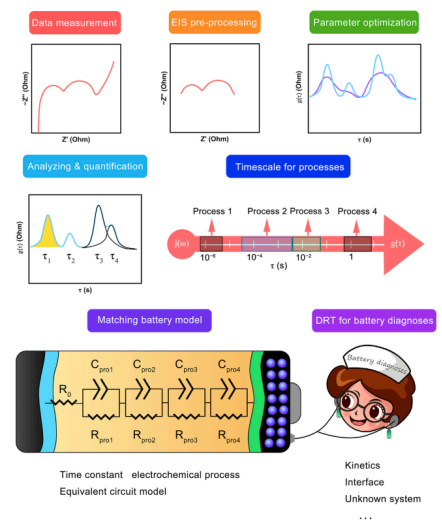
Figure 2 DRT-based timing diagnosis process for battery systems.
DRT diagnosis is initiated by wide-frequency EIS measurements. The accuracy of EIS will determine the results of DRT. Therefore, it is crucial to validate the effectiveness of environmental impact assessment results. Accurate time-domain analysis using DRT requires high-quality EIS data with a high signal-to-noise ratio. Thus, the validity of the environmental impact report data should be verified beforehand. The most commonly used standard is the Kramers-Kronig transformation.
The most important step in DRT is to identify specific electrochemical processes. Simply put, it reveals the timescales of electrochemical processes. Obtaining the DRT plot while ensuring independent time constant peaks is essential.
Interpreting electrochemical processes on a timescale. By distinguishing time scale peaks through the DRT method, different time constants of electrochemical processes can be represented. Therefore, determining the physical significance of different time constants is the core issue of DRT analysis.
Battery modeling and diagnosis. Establishing the relationship between timescale parameters and their real electrochemical processes helps to build a real battery model based on timescales. Identifying different electrochemical processes on a timescale and quantifying their evolution through the DRT method can enable analysis of unknown battery systems, internal evolution monitoring, ECM model optimization, etc.
Timescale Properties
Methods for identifying timescale characteristics. Ensuring that the real processes are based on timescales is the core issue of DRT analysis. On one hand, timescales are determined based on relevant theories and experimental results. On the other hand, experiments need to be designed to determine the physical significance of specific time constants. After using appropriate parameters or algorithms, pseudo-peaks can be suppressed. Peak identification can then be achieved, as shown in the workflow diagram of Figure 3.
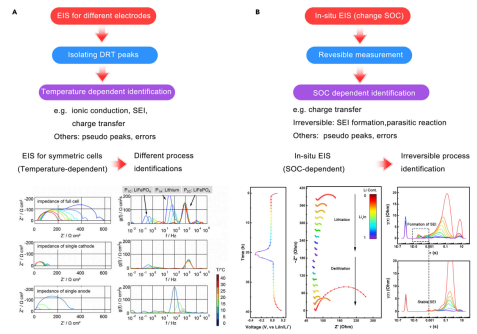
Figure 3 Determining DRT peaks based on temperature-dependent routes and SOC-dependent routes.
Separating electrochemical processes. In the battery configuration view, electrochemical processes mainly include responses based on the electrolyte, anode, cathode, and accessories. In the battery configuration view, measuring a symmetric battery assembled only by the anode or cathode is the basis for separating anode, cathode, and electrolyte responses.
Identifying static/dynamic and reversible processes. Anode or cathode processes can be static or dynamic. Dynamic processes mean that electrochemical processes can be influenced by the charge state. In contrast, static processes remain stable during charging or discharging. SOC-dependent processes can help distinguish between dynamic and static processes. Therefore, half-cells are widely used for SOC-dependent analysis.
This analysis method can achieve analysis of single electrode behavior in half-cells. By using DRT combined with GEIS to control SOC, the formation of SEI and charge transfer processes in graphite anodes can be revealed.
Consulting the timescale dictionary. Timescale analysis based on DRT has gradually accumulated time constants in battery systems. With the enrichment of the timescale dictionary, EIS derived from DRT can be completed quickly.
The time constants of typical electrochemical processes. The fundamental processes in batteries mainly include conductive processes, charging processes based on transfer, physical contact, and diffusion processes (Figure 4A). The total timescale distribution of different kinetic processes is shown in Figure 4B.
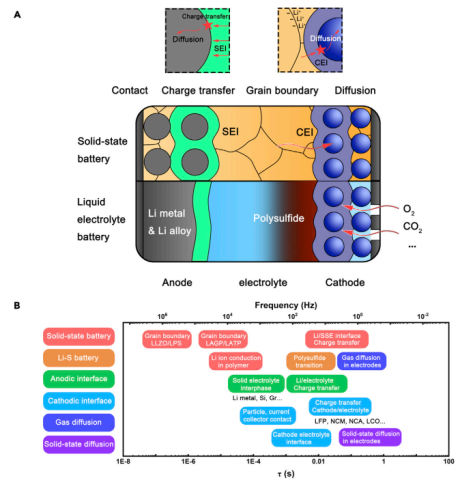
Figure 4 Typical kinetic processes and related time constants of various batteries.
Potential Applications of Timescale Diagnosis
Building electrochemical models. Reasonable interpretation of EIS requires constructing an accurate electrochemical model. The DRT method can distinguish kinetic processes, greatly avoiding subjective judgments. The DRT method helps deepen the understanding of electrochemistry in special battery systems (such as Li-S batteries). As shown in Figure 5, Risse et al. analyzed the time constants and resistances of polysulfides. The charge transfer time range is from 10-2 to 1 s. Full Li-S batteries can also be studied under different SOC states to identify and quantify other processes. In operational Li-S batteries, Soni et al. utilized DRT to ensure the dynamic processes of polarization, which involve eight physical processes such as inter-particle contact, double-layer capacitance, SEI, tri-electrode charge transfer, polysulfide, and lithium-ion diffusion. Research on DRT in full batteries demonstrates that DRT helps bridge the performance of each timescale with the battery state, benefiting not only mechanism research but also practical applications.
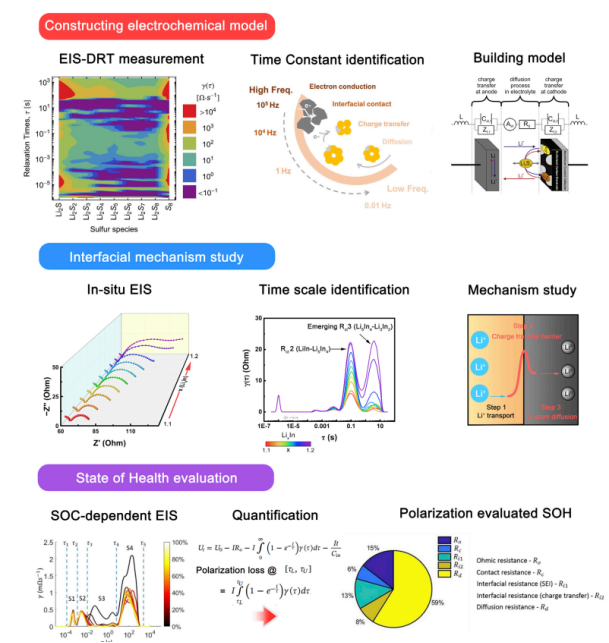
Figure 5 Application of DRT in Li-S battery electrochemical models, interface studies, and SOH evaluation based on DRT quantified polarization losses.
Interface mechanism research. Interface evolution includes the formation of SEI, CEI, charge transfer evolution, the formation of solid-liquid interfaces in mixed batteries, and the identification of solid-solid interfaces, all exhibiting different characteristics. Interface evolution is always accompanied by a series of comparisons of EIS methods. Using EIS can detect the formation of SEI on graphite anodes, and DRT can analyze the formation process of SEI. At different SOC stages, the evolution of timescales can be achieved under different half-electrode modes. Timescale-based analysis clearly shows SOC-induced evolution in full batteries. However, some timescale-based properties remain elusive. Timescale identification can provide high-resolution EIS interpretation. Solid-solid interfaces can produce significant ionic capacitive behavior. Ionic conduction at grain boundaries, bulk, and contact interfaces can be identified on a timescale. Based on the above results, an ECM can ultimately be constructed. As shown in Figure 5 (middle image), after in-situ EIS evaluation, the charge transfer evolution caused by phase transitions from LiIn, Li5In4 to Li3In2 becomes more pronounced. After clearly understanding the charge transfer and diffusion processes, a kinetic model of the anode lithiation process can be constructed.
Emerging Applications of Timescale Analysis
From one-dimensional DRT to multi-dimensional DRT, conventional DRT is limited by data accuracy and regularization hyperparameters. One major reason is that EIS is only interpreted as a function of frequency (one-dimensional data, only relative to frequency). Therefore, DRT interpreted solely by frequency is called 1D-DRT. If the EIS spectrum is measured under different external environments (such as temperature, different battery stages, and other experimental conditions), forming an EIS dataset, its limitations will be relaxed from a single frequency to multi-dimensional experimental conditions. Furthermore, DRT analysis can connect data with frequency dependencies. Multi-dimensional DRT is expected to improve accuracy and expand new application functionalities. Mertens et al. first proposed 2D-DRT, introducing a new temperature dimension, reducing the uncertainty of EIS, and helping to improve resolution. Quattrocchi et al. constructed deep neural networks (DNNs) to examine, interpret, and clarify the dependencies of EIS from multiple experimental conditions. 2D, 3D, and 4D experimental data were obtained in different SOFC batteries and perovskite solar cell electrochemical systems, demonstrating the versatility of deep DRT. Deep DRT shows promise in analyzing the impedance of various batteries, supercapacitors, and other complex electrochemical systems. Therefore, multi-dimensional DRT can be used for monitoring and identifying battery states in the battery industry, achieving SOC assessments, etc.
Data-driven modeling foundation. Timescale deconvolution (DRT) is a physical method for vectorizing EIS data (Figure 6). Batteries in different states (such as SOC, aging time, cycle life, remaining capacity, etc.) will have specific impedance characteristics. These states are viewed as a set of ensured targets. The measured EIS will illustrate two dimensions, namely a series of timescales and timescale-related impedance strengths. Therefore, specific batteries can be described using vectors of timescale, impedance, and battery state. Many specific batteries can form a dataset, and after data-driven machine learning, appropriate estimation models can be established.
Figure 6 Modeling batteries through DRT, providing data-driven sources for machine learning-based AI predictions.

Conclusion and Outlook
Timescale-based analysis provides an opportunity to separate coupled dynamic information in the battery’s “black box.” Studying its internal dynamic characteristics is a powerful approach. EIS can identify different dynamic processes by integrating DRT. In short, DRT-based timescale analysis can significantly promote fundamental understanding of electrochemistry, with the following unique advantages: efficient and accurate analysis, providing new insights on timescales, offering strong references for ECM, accurate measurement of EIS, accuracy of DRT algorithms, determining the true meaning of specific time constants, combining DRT models with DDT and DDC, extending one-dimensional DRT to multi-dimensional DRT, and data-driven battery analysis applications.

References
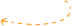
The Timescale Identification Decoupling Complicated Kinetic Processes in Lithium Batteries. (Joule. 2022, DOI: 10.1016/j.joule.2022.05.005)
Original Link:
https://www.sciencedirect.com/science/article/pii/S254243512200232X
 Fresh Power Submission Channel (Scan)
Fresh Power Submission Channel (Scan)