Introduction
Using amphiphilic block copolymers as soft templates, the controlled cooperative assembly between inorganic nanomaterials (such as inorganic metal salts, nanoparticles, and polyoxometalates (POMs)) and organic amphiphilic block copolymers through interfacial-induced co-assembly and “bottom-up” assembly is an effective approach to create multifunctional mesoporous nanomaterials. Mesoporous metal-based materials with rich porosity and tunable pore structures not only exhibit unique properties of metal-based materials (such as metal oxides, nitrides, carbides, etc.) and nanoscale effects but also possess advantages in mass transfer and diffusion as mesoporous materials. Research on the synthesis, design, assembly, and regulation of mesoporous metal-based nanomaterials is currently a significant focus in the field of porous materials, particularly for applications in catalysis, gas sensing, and energy conversion.
Summary of Findings
Recently, Professor Deng Yonghui from Fudan University (corresponding author) and his team published a review titled “Recent advances in amphiphilic block copolymer templated mesoporous metal-based materials: assembly engineering and applications” in the prestigious international chemistry journal Chemical Society Reviews, with the first author being doctoral student Zou Yidong from the 2017 cohort at Fudan University. The article systematically summarizes the design and synthesis of amphiphilic block copolymers and the advantages of soft template-directed assembly for synthesizing mesoporous metal-based materials. It compares the characteristics and advantages of three main types of metal precursors (inorganic salts, nanoparticles or clusters, and polyoxometalates (POMs)) in the synthesis of mesoporous materials, highlights research progress in the preparation and structural regulation of different types of mesoporous metal-based materials, and discusses the application prospects and future challenges faced by mesoporous metal-based materials.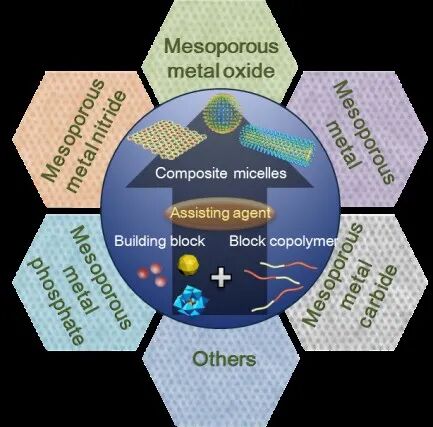
Visual Guide
1. IntroductionMesoporous materials have attracted significant attention due to their unique pore structures, high porosity, high specific surface area, and excellent mass transfer/diffusion pathways. They are widely used in catalysis, gas sensing, environmental remediation, biomedical applications, and energy conversion and storage. Over nearly half a century of development, the focus of mesoporous materials has shifted from initially inorganic non-metallic materials (such as mesoporous silica and mesoporous carbon) to more functionally rich mesoporous metal-based materials (such as semiconductor metal oxides and carbides). In contrast, mesoporous metal-based materials possess a broader application value due to their combined properties of mesoporous and metal-based materials, especially in industrial catalysis and sensor applications. However, the synthesis of mesoporous metal-based materials has faced various challenges and bottlenecks, from the initial hard template synthesis techniques to the currently popular soft template synthesis techniques, each with its own advantages and disadvantages.
As researchers delve deeper into the controllable synthesis and assembly of organic polymers, soft template synthesis methods based on amphiphilic polymer co-assembly technology have rapidly developed. Traditionally, due to their composition and low thermal stability, commercially available amphiphilic polyether block copolymers (such as F127, P123, etc.) used as soft templates in the synthesis of mesoporous metal-based materials often lack good skeletal stability and regular ordered pore structures. With the ongoing pursuit of high-stability crystalline mesoporous metal-based materials, new amphiphilic block copolymers rich in sp2 carbon (such as PEO-b-PS, PS-b-P4VP, etc.) have provided new opportunities for designing high-crystallinity mesoporous metal-based materials due to their relatively high thermal decomposition stability and high residual carbon content in inert atmospheres. By utilizing interactions between inorganic precursors and amphiphilic block copolymers, such as electrostatic interactions, hydrogen bonding, van der Waals forces, or coordination interactions, cooperative co-assembly of organic-inorganic components can be achieved to form ordered mesoscopic composite structures. Through in-situ transformation and subsequent selective removal of the template, the target functional mesoporous materials can ultimately be obtained. After decades of development, significant progress has been made in constructing mesoporous metal-based materials using amphiphilic block copolymers as templates. Researchers can achieve precise control over pore size, pore wall thickness, and specific surface area by altering the composition of block copolymers (types, lengths, and degrees of polymerization of hydrophilic and hydrophobic segments). By changing the type and ratio of organic solvents, controllable adjustments of pore structure, crystal phase, and active surface can be achieved. Additionally, by modifying the template removal methods, such as calcination or ozone plasma treatment, adjustments to the crystallinity and grain size of mesoporous materials can be realized.
Furthermore, in addition to regulating the properties of amphiphilic block copolymers and precursors, organic solvents, and template removal methods, some research progress has also been made in component design, doping, and composite materials, especially for specific functional applications of multi-component mesoporous metal-based materials and heterojunction mesoporous materials. These newly functionalized and structured mesoporous metal-based materials exhibit outstanding application potential, primarily involving gas sensing, catalysis, adsorption, drug release, and energy storage and conversion.
Figure 1 Assembly engineering, structure-activity relationship, and potential applications of mesoporous metal-based materials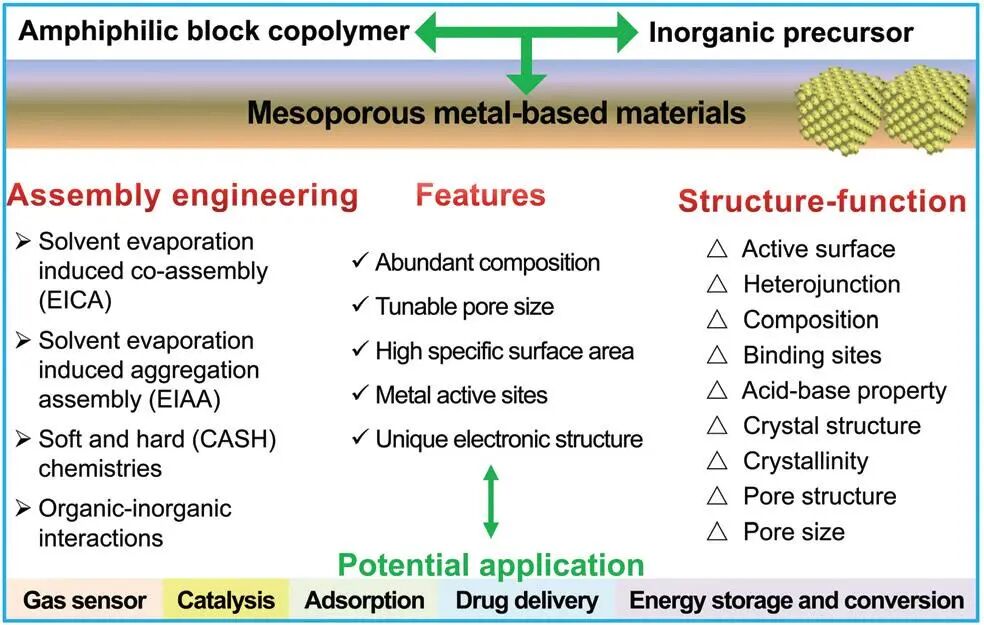 2. Co-assembly of Block Copolymers with Metal Inorganic Salts
2. Co-assembly of Block Copolymers with Metal Inorganic Salts
Metal inorganic salts play an irreplaceable role as important precursors in the synthesis of mesoporous metal-based materials, primarily due to the ease of hydrolysis and condensation reactions of inorganic metal salts in organic solvents, allowing metal ions to easily co-assemble with amphiphilic block copolymers during a series of reactions. In recent years, a series of novel synthesis strategies have been developed for the assembly between metal inorganic salts and block copolymers, including solvent evaporation-induced co-assembly (EICA), carbon support strategies, ligand-assisted strategies, and Resol crosslinking-assisted strategies, particularly for the design and synthesis of bimetallic oxides, solid solutions, and heterojunctions. By controlling the assembly environment, such as temperature, pH, and ion types, efficient hybridization of multi-components has been achieved, leading to the development of a series of mesoporous metal-based materials with high crystallinity, high specific surface area, and abundant active sites.
Figure 2 Directed assembly of ordered mesoporous Ce-Zr solid solution nanomaterials and Pt-loaded hybrid materials using amphiphilic block copolymer PEO-b-PS as a template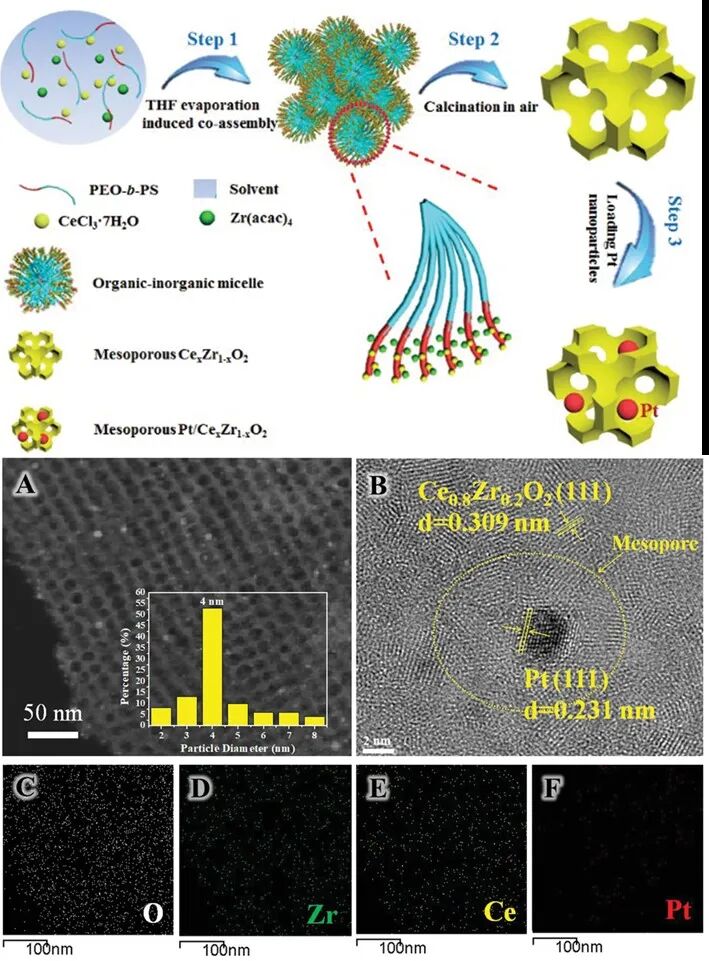
3. Co-assembly of Block Copolymers with Metal Nanoparticles or Clusters
In addition to common inorganic metal salts, pre-crystallized ultrafine metal-based nanoparticles or nanoclusters can also serve as precursors. These ultrafine nanoparticles, synthesized through methods such as thermal decomposition, alcoholysis, or solvothermal processes, typically have sizes <10.0 nm and often exhibit some crystallinity after thermal treatment. However, due to the exposed or lipophilic surfaces of some nanoparticles, surface modification is required, primarily through ligand exchange using organic ligands or small inorganic hydrophilic ligands. This is followed by the assembly of nanoparticles with amphiphilic block copolymers through hydrogen bonding, electrostatic interactions, or van der Waals forces, constructing ordered mesoporous metal-based composite materials with certain crystallinity and particle-like frameworks. Compared to inorganic metal salts, nanoparticles as precursors can effectively avoid uncontrollable hydrolysis processes and allow for gentle removal of templates, but the synthesis and surface modification processes of nanoparticles are relatively stringent, thus each type of precursor has its own advantages and disadvantages.
Figure 3 Schematic diagram of co-assembly and interactions between amphiphilic block copolymers and ligand-modified nanoparticles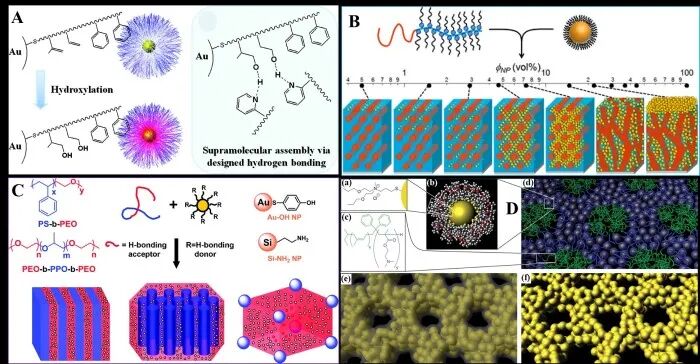
4. Co-assembly of Block Copolymers with Polyoxometalates
Polyoxometalates (POMs) are a class of nanoscale stable transition metal oxide clusters with rich compositions, sizes, charges, and shapes. Common Keggin-type POMs can release H+ and negatively charged metal groups in organic solvents, which can easily co-assemble with protonated amphiphilic block copolymers through hydrogen bonding or electrostatic interactions, constructing ordered mesoporous structures or mesoporous nanowire arrays with rich compositions. This precursor does not require hydrolysis/condensation reactions during assembly, thus simplifying the assembly environment. Notably, POMs, due to their multiple components, often contain inorganic non-metals (such as Si, P, etc.) and metals (such as W, V, and Mo), allowing for the convenient one-step synthesis of multi-component metal-based materials and enabling in-situ doping of non-metal components, significantly enhancing the overall performance of hybrid materials.
Figure 4 Co-assembly of amphiphilic block copolymer PB-b-P2VP and H3PMo to construct ordered mesoporous metal oxides and carbides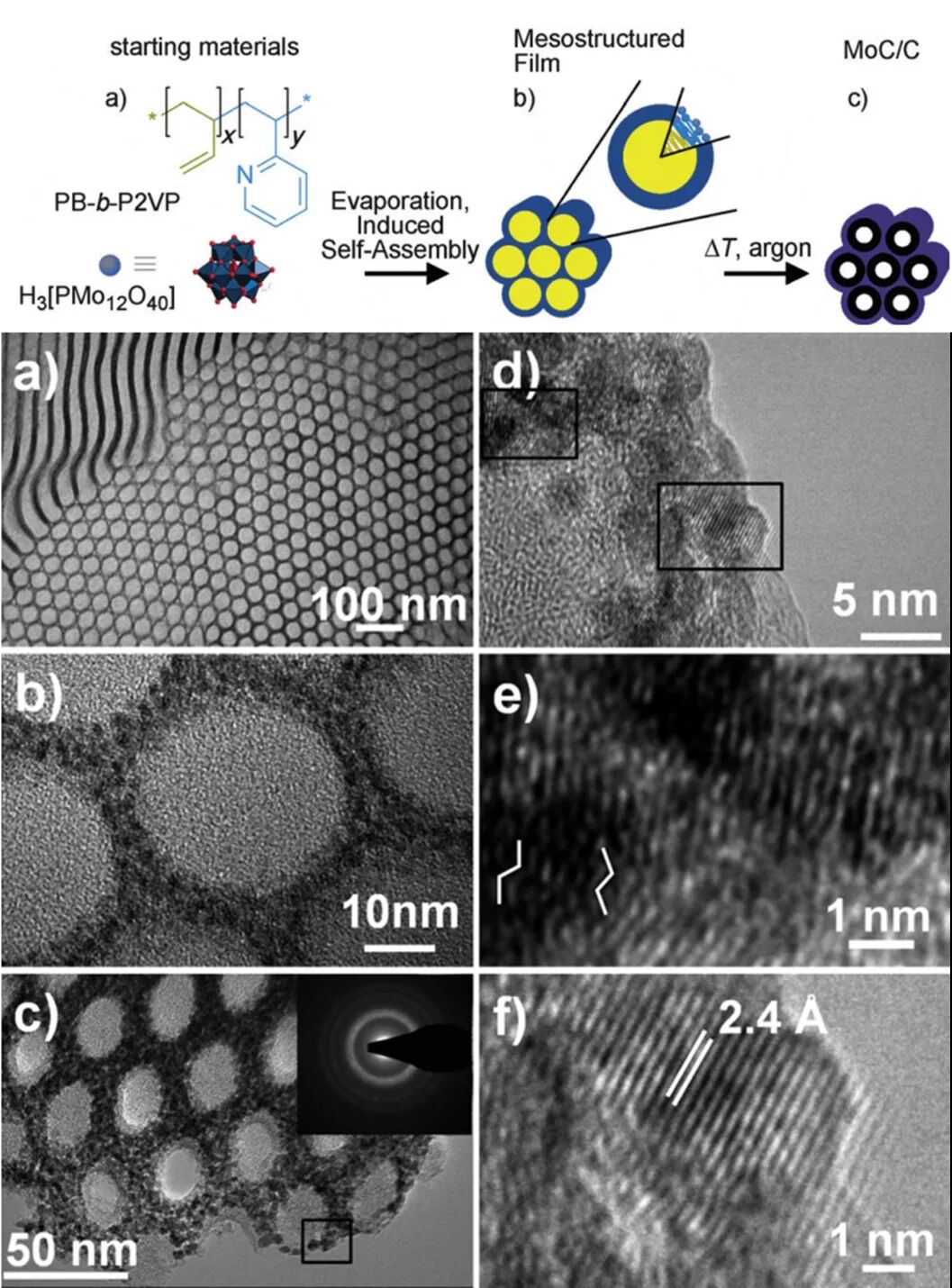
5. Types of Mesoporous Metal-Based Materials
With the use of nanoparticles and polyoxometalates as inorganic precursors for constructing mesoporous metal-based materials, the limitations of inorganic metal salt precursors in synthesizing special mesoporous metal-based materials have been addressed, leading to the rapid development of various types of mesoporous metal-based materials, including: mesoporous metal elemental materials, mesoporous metal oxides, mesoporous metal carbides, mesoporous metal nitrides, and other mesoporous metal-based materials.
For example, mesoporous metal elements, particularly precious metals, have garnered significant attention due to their outstanding catalytic activity. However, the synthesis of mesoporous precious metals has long been a major challenge in the field of mesoporous materials. With the assistance of supramolecular chemistry and interfacial assembly chemistry, researchers have successfully used low molecular weight and high molecular weight amphiphilic block copolymers to guide the assembly of ordered mesoporous structures of mesoporous metals, including mesoporous Pt, mesoporous Pd, mesoporous Rh, mesoporous Au, and mesoporous Pt-Au alloys.
Figure 5 (A) Co-assembly of block copolymer PEO-b-PS with HAuCl4 to construct mesoporous Au films; (B) Co-assembly of block copolymer PEO-b-PMMA micelles to synthesize mesoporous Rh nanoparticles.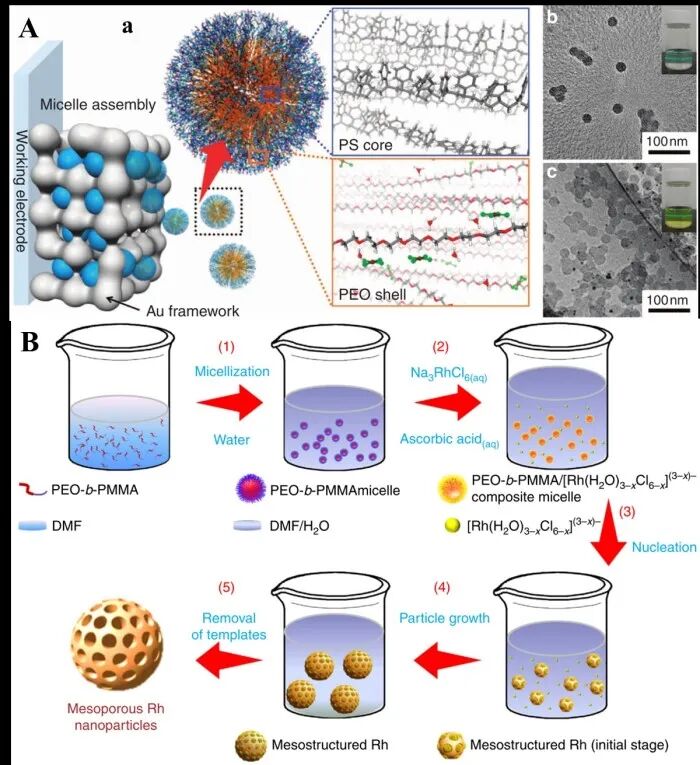
Mesoporous metal carbides are a type of special material formed by embedding C atoms into metal sites, which exhibit high thermal stability. In contrast, mesoporous metal carbides have outstanding application value in electrocatalysis and energy conversion, but their preparation conditions are extremely stringent, often requiring high-temperature carbonization (typically above 1100 K). With the assistance of amphiphilic block copolymers, typical mesoporous metal carbides have gradually been developed, such as mesoporous WC, Mo2C, and TiC.
Figure 6 Synthesis of ordered mesoporous TiC using commercial small molecule block copolymer F127 as a soft template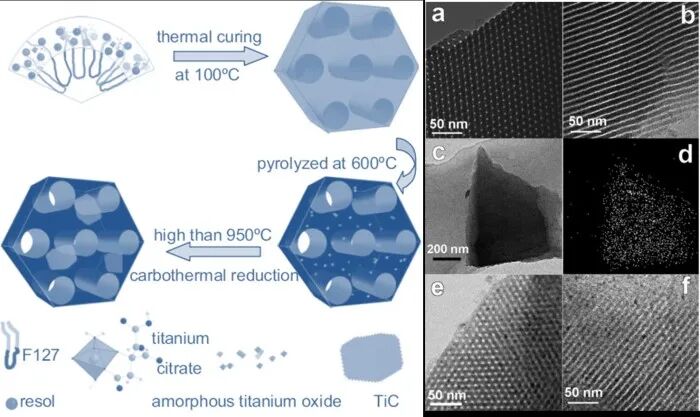
In contrast, the synthesis of mesoporous nitrides is similar to that of mesoporous carbides, but the synthesis conditions for mesoporous carbides are more stringent. It not only requires high calcination temperatures (usually up to 700 °C) to nitrify the synthesized mesoporous metal oxides but also necessitates filling the high-temperature reaction chamber with sufficient NH3 atmosphere for nitrification. Researchers have employed different amphiphilic block copolymers to co-assemble and pre-synthesize metal oxides with highly stable mesoporous skeletal structures, which are then nitrified to convert them into mesoporous nitrides, commonly including TiN and NbN.
Figure 7 (A) Schematic diagram of the synthesis of ordered mesoporous TiN-C composites through co-assembly of commercial amphiphilic block copolymer F127 with TiCl4 followed by nitrification; (B) Ordered mesoporous NbN synthesized using block copolymers ISO-64 k and ISO-86 k.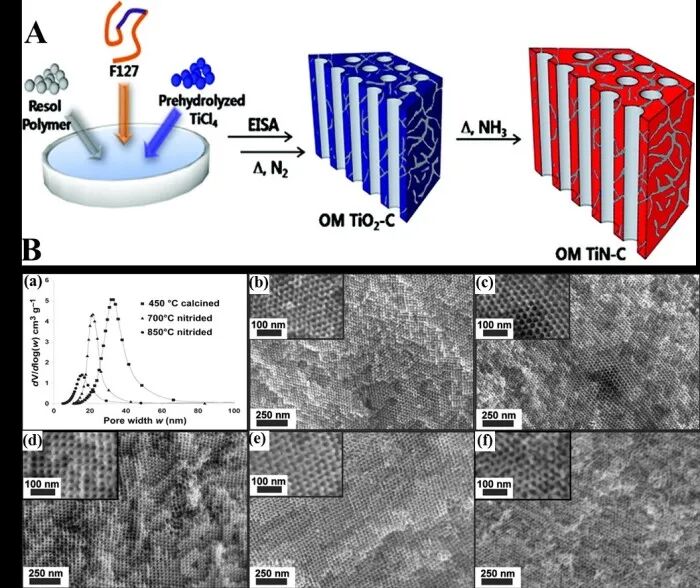
6. Applications of Mesoporous Metal-Based Materials
Mesoporous metal-based materials have demonstrated enormous application potential in gas sensing, catalysis, environmental remediation, biomedical applications, and energy conversion. Compared to bulk non-porous materials, the excellent pore structure, adjustable pore size, and high specific surface area of mesoporous metal-based materials provide abundant active sites, reaction surfaces, and mass transfer/diffusion pathways during reactions.
For instance, in gas sensing, our research group has designed and synthesized a series of mesoporous semiconductor metal-based materials with high porosity, long-range ordered pore channels, high specific surface area, and high crystallinity, such as In2O3, WO3, Pt-doped WO3, CoOx, WO3/NiO, Fe2O3, Pd-doped In2O3, SnO2, NiO, ZnO, etc. Whether as single-component metal oxides or hybrid materials after element doping or compounding, they exhibit superior overall gas sensing performance compared to similar bulk materials, and these mesoporous metal-based materials will play an extremely important role in the development of high-performance smart gas sensors.Figure 8 Study of the sensing performance of ordered mesoporous ZnO hybrid materials doped with SiO2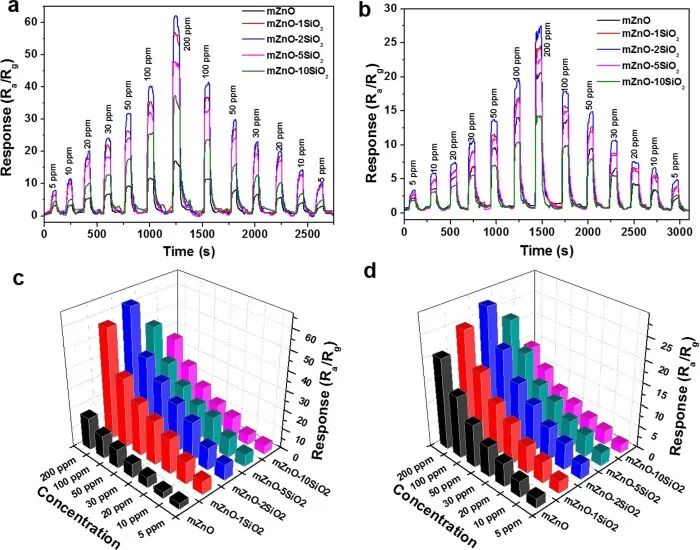
Conclusion and Outlook
High porosity, high crystallinity, and high activity functional mesoporous metal-based materials are a shining gem in the field of nanomaterials research, bringing new hope and brightness to applications in catalysis, sensing, and energy. The method of using amphiphilic block copolymers as structural directing agents for synthesis has shown good plasticity, compatibility, and flexibility, achieving remarkable progress in the synthesis of mesoporous metal-based materials. However, related synthesis research still mainly remains at the mesoscopic scale, while studies on material properties closely related to performance, such as crystal plane control, pore wall microstructure regulation, and defect control, need further strengthening and deepening.Therefore, future research needs to develop new synthesis methods that can achieve rational design and precise synthesis of materials at smaller scales, and elucidate the intrinsic relationship between material properties and structures at mesoscopic, sub-nanometer, and atomic scales, continuously developing mesoporous metal-based materials with controllable structures and excellent performance, fully leveraging the advantages of material defects, crystal planes, heterojunctions, and multi-components, and exploring atomic-level assembly behaviors and mechanisms. We believe that with the intersection and penetration of multiple research fields, the synthesis, assembly, regulation, and application of mesoporous metal-based materials will usher in a new wave of research enthusiasm.
Reference link: Recent advances in amphiphilic block copolymer templated mesoporous metal-based materials: assembly engineering and applications (Chem. Soc. Rev., 2020, 49, 1173-1208. DOI: 10.1039/C9CS00334G)
Corresponding Author Profile
Professor Deng Yonghui obtained his bachelor’s degree in inorganic chemistry from Nanchang University in 2000; his Ph.D. in polymer chemistry and physics from Fudan University in 2005; conducted postdoctoral research in the group of Academician Zhao Dongyuan at Fudan University from 2005 to 2007, and then stayed at the university as an associate professor; was a visiting scholar at the University of California, Berkeley from 2009 to 2010; and has been a professor since 2011. Professor Deng mainly engages in the synthesis of functional porous materials and their applications in intelligent gas sensing and catalysis, proposing new concepts and methods for the cooperative co-assembly of organic amphiphilic block copolymers with various inorganic precursors, creating a series of new functional mesoporous materials, and has published over 140 papers in journals such as Nature Materials, J. Am. Chem. Soc., Angew. Chem. Int Ed., and Adv. Mater. (cited over 12,000 times, H-index of 57 (Google Scholar)). He has been invited to publish several review papers in top review journals such as Acc. Chem. Res, Chem. Soc. Rev., and Nano Today. He has received numerous honors, including the first-class Natural Science Award from the Ministry of Education (second contributor, 2017), the second-class Natural Science Award from the Ministry of Education (first contributor, 2014), the first batch of Young Changjiang Scholars from the Ministry of Education (2015), the second batch of National Ten Thousand Talents Program Young Top Talents (2015), the National Excellent Young Fund (2014), Shanghai Youth Science and Technology Talent (2014), Shanghai Shuguang Scholar (2013), and Shanghai Youth Science and Technology Star (2008, 2012 Star Tracking Program). He has been selected for the Elsevier China Highly Cited Scholars list for five consecutive years from 2014 to 2018 (Materials Science); recognized as one of the 2014 Emerging Investigators by the journal J. Mater. Chem. A (global 35 people). He serves as an executive member of the Porous Materials Branch of the Chinese Materials Research Society; a director of the Shanghai Chemical and Chemical Engineering Society; a director of the Shanghai Military-Civilian Integration Development Research Association; a senior member of the Chinese Materials Research Society; a member of the Chinese Chemical Society; a director of the Nanobiology Branch of the Chinese Biophysical Society; and the executive deputy editor of Chinese Chemical Letters (IF: 3.84); and an international project review expert for the Australian Research Council (ARC).