
Original link:https://pubs.acs.org/doi/10.1021/jacs.4c14511
First authors:Yaling Jiang and Peimeng Qiu
Corresponding authors:Peng Li and Shengli Chen
Corresponding author affiliation:Wuhan University, China
【Core Innovation Point】
The authors systematically elucidate for the first time the impact of oxygen affinity (the affinity of metal surfaces for *OH) on the kinetics of the alkaline hydrogen evolution reaction (HER), which fundamentally arises from the regulation of the electrode’s zero charge potential (PZC), thereby affecting the interfacial electric field strength, hydrogen bond network of water molecules, interfacial environmental rigidity, and proton transfer dynamics, rather than merely due to the traditional bifunctional mechanism or simple electronic effects. The results indicate that HER activity, the strength of the interfacial water hydrogen bond network, O-H stark slope, electrode PZC, etc., all exhibit a significant volcano-type dependence on surface oxygen affinity.
【Research Background, Existing Research Methods, and Challenges】
Under alkaline conditions, the HER/HOR kinetics are two orders of magnitude slower compared to acidic environments, which is a bottleneck for the development of AEM fuel cells and AEM electrohydrogen production technologies. Surface oxygen affinity regulation is widely regarded as an important strategy to effectively improve alkaline hydrogen reaction kinetics, and this strategy is not limited to hydrogen reactions but has been found to be generally applicable to various O bond-related electrocatalytic processes (such as oxygen evolution/reduction reactions, CO2 reduction reactions, and alcohol oxidation reactions, etc.). Currently, the mechanistic studies of this regulatory strategy mainly focus on the “catalyst-intermediate” interactions (such as bifunctional mechanisms: oxygen affinity regulates *OH adsorption strength, thereby affecting the energy barrier for water dissociation/formation; electronic effect mechanisms: changes in electronic structure and fine-tuning of *H adsorption), neglecting the direct regulation of the electrode-electrolyte interfacial microenvironment on the reaction rate..
【Core Scientific Question】
How to understand the regulation of surface oxygen affinity on interfacial electrochemical structure and its impact on hydrogen evolution kinetics under alkaline conditions? And whether there is a unified law to describe it?
【Specific Design】
The authors modified the surface of a Pt film electrode using electrochemical deposition to create four representative metal atoms: Mo, Ru, Rh, Au, forming a series of PtM (M=Mo, Ru, Rh, Au) film electrodes. By controlling the deposition parameters, two types of electrodes with low coverage (PtM-low) and high coverage (PtM-high) can be obtained, providing a model system for systematic research. By quantifying the HER activity of modified Pt film electrodes with different surface oxygen affinities (LSV+CV), and using attenuated total reflectance-surface-enhanced infrared absorption spectroscopy (ATR-SEIRAS), the interfacial water molecules, hydrogen bond network, and electric field environment on the electrode surface during the reaction (at different potentials) were detected, and finally, the intrinsic properties of the interface were clarified in conjunction with DFT and AIMD. The results indicate that HER activity, the strength of the interfacial water hydrogen bond network, O-H stark slope, electrode PZC, etc., all exhibit a significant volcano-type dependence on surface oxygen affinity.
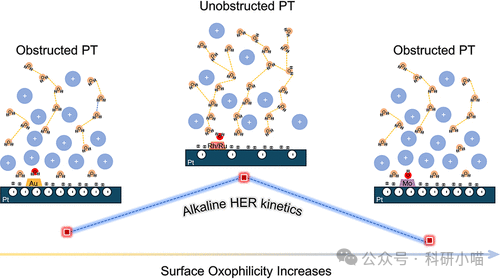
Figure 1. Schematic diagram of the origin of surface oxygen affinity regulation on alkaline HER kinetics
【New Information and Understanding】
LSV curves indicate that the HER activity of Pt electrodes modified with different oxygen-affinity metals (Mo, Ru, Rh, Au) has changed significantly (normalized to geometric area, avoiding overestimation of activity due to reduced Pt active sites from deposition or the stronger reactivity of the deposited transition metals themselves). Experimental results show that when Ru and low coverage Rh modify the Pt electrode, the HER activity is significantly enhanced, exhibiting higher current density and lower overpotential; whereas when Mo and Au modify the Pt electrode, the HER activity decreases, and the current density drops. This result presents a clear volcano-type dependence of Pt surface oxygen affinity on HER activity.
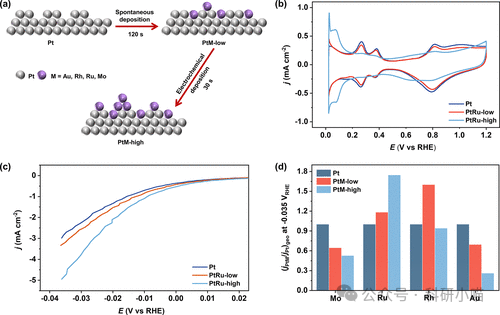
Figure 2. Changes in alkaline HER activity of Pt film electrodes caused by different oxygen-affinity metal deposits
Furthermore, the authors used density functional theory (DFT) to calculate the OH adsorption free energy (ΔGOH). By correlating the dependence of HER activity enhancement and reduction with the OH adsorption free energy (ΔGOH, i.e., oxygen affinity), a volcano curve can be constructed: that is, catalysts with moderate oxygen affinity (such as Ru, low coverage Rh modification) are optimal, while too strong or too weak oxygen affinity (such as Mo, Au modification) are detrimental to HER.
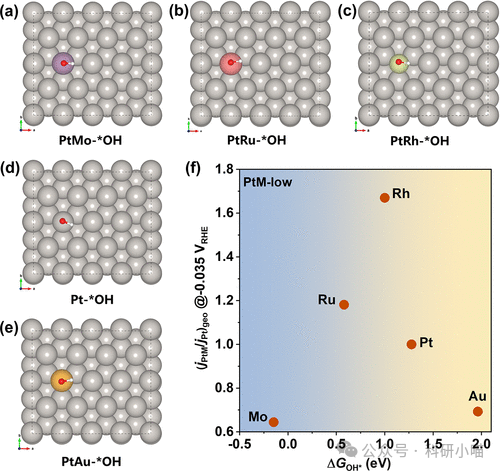
Figure 3. DFT calculations of OH adsorption free energy on Pt(111) surface modified with different oxygen-affinity metals
Secondly, the authors used in situ ATR-SEIRAS technology to probe the interfacial water structure on the modified Pt film electrodes, analyzing the role of surface oxygen affinity in regulating the electrode surface charge and double layer structure to understand the volcano dependence of alkaline HER activity on surface oxygen affinity. In situ ATR-SEIRAS experiments showed that the H-O-H bending vibration peak (δH-O-H, δH-O-H vibration frequency is very sensitive to the strength of hydrogen bonds in water molecules, such as stronger hydrogen bonds—δH-O-H blue shift) located at ~1625 cm-1 and the O-H stretching vibration peak (νO–HνO–H ranging from 3000 to 3600 cm-1 (the Stark shift of νO–H with changes in potential can indicate the flexibility/rigidity of the interfacial environment, electric field strength, and the ease of water molecule reorganization, etc.). For δH-O-H, moderately oxygen-affinity metals (such as Rh, Ru) modified Pt electrodes exhibited significant blue shift and increased absorption intensity, indicating a more stable and ordered structure of the interfacial water hydrogen bond network. In contrast, strong oxygen-affinity (metal Mo) or weak oxygen-affinity (metal Au) modifications caused this vibration band to red shift and peak shape to weaken, reflecting the destruction of the hydrogen bond system of water molecules and a decrease in order. For νO–H, with changes in applied potential, the νO–H peak of Rh and Ru modified samples showed greater red shifts, and the Stark shift was significantly enhanced, indicating that the interfacial water’s response capability to the electric field of the electrode surface was enhanced, and the interfacial environment was more flexible, facilitating the reorganization of water molecules and proton transfer; conversely, during Mo and Au modifications, the displacement of the νO–H peak was not significant, limiting the flexibility of interfacial water and weakening the synergistic effect between the interfacial electric field and hydrogen bond network. These spectroscopic feature parameters exhibited a consistent volcano-type dependence with HER activity, strongly confirming the microscopic mechanism of catalyst interfacial oxygen affinity regulation on catalytic performance.
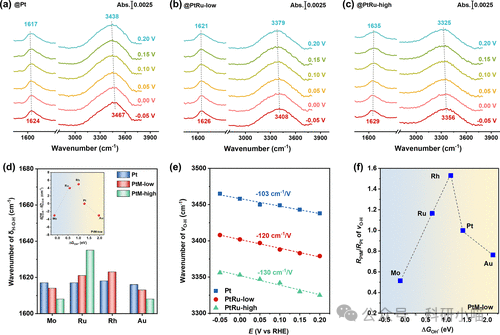
Figure4. ATR-SEIRAS results of Pt film electrodes with different oxygen-affinity metal deposits as a function of potential
Finally, to further reveal the molecular mechanism of surface oxygen affinity on the reconstruction of the electrocatalytic interfacial microenvironment, the authors utilized density functional theory (DFT) and ab initio molecular dynamics (AIMD) simulations to conduct a multi-dimensional theoretical analysis of the microscopic structure and dynamic behavior of Pt surface catalysts modified with different metal atoms (Mo, Ru, Rh, Au) at the water solution interface. Here, it is necessary to introduce a prerequisite understanding: the difference in HER activity under acidic and alkaline conditions originates from the poor connectivity of interfacial water reorganization and/or hydrogen bond networks in the double layer, leading to slow HER kinetics (i.e., under alkaline conditions, the HER reaction potential is far below PZC vs. RHR, thus the electric field forces water molecules to redirect, resulting in enhanced rigidity of the hydrogen bond network, making reorganization difficult, limiting water dissociation/proton or hydroxyl migration, and greatly restricting HER kinetics). Theoretical calculations and simulation results indicate that surface oxygen affinity dominates the interfacial environment by altering the electrode PZC. Mo and Au modifications increase PZC (Mo leads to *OH enrichment, Au leads to too weak oxygen affinity (*OH is difficult to adsorb and reduces effective orientation of water molecules)), causing the working potential to move far from PZC, resulting in a stronger electric field and rigid interface, making water dissociation and proton transfer difficult, significantly decreasing HER rate; while Ru and Rh modifications lower PZC, creating flexible proton transfer channels, which facilitate the directional reorganization of water molecules and efficient proton transfer processes, thereby significantly enhancing HER kinetic performance. This explanation collectively constitutes the fundamental physical mechanism origin of the volcano-type variation of alkaline HER catalytic activity with oxygen affinity.
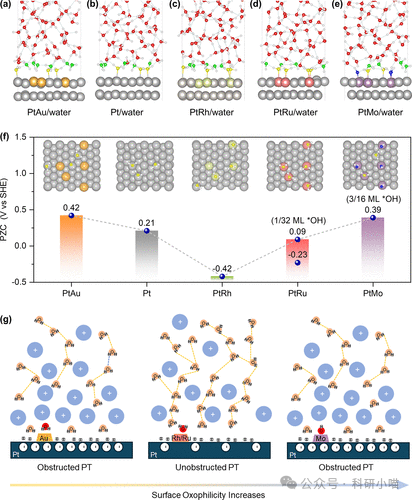
Figure5. Theoretical calculations indicate that surface oxygen affinity dominates the interfacial environment by altering the electrode PZC, thereby affecting HER catalytic activity
【Thoughts】
This study reveals the deep relationship between oxygen affinity, PZC, and HER kinetics, not only answering the classic question of “how does catalyst oxygen affinity affect HER,” but also providing profound insights into the coupling mechanism of “electrode-interface-microenvironment-reaction kinetics” for the entire field. It is extremely valuable for us to learn from and reference!!!