

The liver is one of the most metabolically active organs in the body and is crucial for maintaining the internal environment balance. Its main functions include the secretion of endogenous active substances and the transformation or clearance of toxic substances. When liver function is impaired due to factors such as viruses, drugs, or surgery, an artificial liver support system (ALSS) can temporarily replace the liver to clear some toxic substances from the systemic circulation, improve inflammation, and hemodynamics, providing opportunities for liver regeneration or transplantation. Based on the basic physiological functions of the liver, ALSS is designed into two categories: one is the non-biological artificial liver (NBAL) aimed at removing various toxins from plasma; the other is the biological artificial liver (BAL) that mimics the secretion and transformation functions of liver cells. Currently, ALSS can be used for liver failure caused by various reasons, pre-transplant waiting, post-transplant rejection, periods of non-functionality of transplanted liver, persistent itching due to cholestasis, acute drug/food poisoning, etc. Due to certain defects in single-mode ALSS, which cannot simultaneously clear toxic substances and supplement related active substances (proteins, coagulation factors), the clinically widely used system is the combined ALSS. This article elaborates on the clinical applications of combined NBAL and the research progress of combined BAL in recent years, both domestically and internationally.

1Combined NBAL
NBAL is established through blood purification, utilizing external devices to clear various harmful substances from blood using principles such as ultrafiltration, adsorption, osmosis, diffusion, and filtration. In recent years, with the continuous development of membrane technology, NBAL has derived many modes based on the disease characteristics and treatment needs of patients. Currently, the mature NBAL applications in China include plasma exchange (PE)/selective plasma exchange (SPE), molecular adsorption of plasma (MAS), blood/plasma perfusion (HP/PP), blood/plasma dialysis filtration (HDF/PDF), etc. Abroad, the main systems are the molecular adsorption recycling system (MARS) based on albumin dialysis and the Prometheus system for graded plasma separation and adsorption. This author introduces the clinical application progress of combined NBAL based on the recommended combined modes from the domestic “Expert Consensus on Clinical Application of Artificial Liver Blood Purification Technology (2022 Edition)”; its advantages and disadvantages are detailed in Table 1.

1.1 DPMAS+PE
In studies on patients with liver failure, it was found that compared to the PE treatment group alone, DPMAS+PE treatment significantly reduced plasma consumption, improved liver enzymes and coagulation function, and increased survival rates at 28 days and 90 days. In children with acute liver failure undergoing artificial liver support treatment, DPMAS+PE did not change the 28-day survival rate compared to the PE group alone but significantly reduced plasma consumption, thereby reducing the occurrence of adverse events related to artificial liver treatment. DPMAS+PE has also been used to improve clinical symptoms in patients with hyperthyroidism crisis combined with acute liver injury.
1.2 PE+HDF/PDF
Research shows that PE+HDF can increase the range of toxin clearance, alleviate hepatic encephalopathy, electrolyte disorders, renal dysfunction, etc., and is suitable for patients with complications such as hepatorenal syndrome and hepatic encephalopathy. In patients with Kawasaki disease combined with hemophagocytic lymphohistiocytosis and severe immunological conditions, the application of HDF+PE can significantly improve symptoms and prognosis. PDF is a combination mode of PE and continuous slow blood dialysis filtration, used for patients with septic multi-organ failure, which can significantly reduce 28-day mortality. However, in patients with late-stage liver failure combined with multi-organ failure, continuous plasma dialysis filtration is safer and more effective than PDF. PDF can reduce pro-inflammatory factor levels in patients with macrophage activation syndrome and improve multi-organ dysfunction in exertional heat stroke.
1.3 HDF+DPMAS, PE+PP+HDF, PDF+PP
These three modes are based on blood dialysis filtration, with large blood volumes and long treatment times, effectively improving patients’ systemic inflammatory response syndrome/multiple organ dysfunction syndrome status. Among them, HDF+DPMAS can be used for patients with severe infections and multiple organ dysfunction due to a lack of blood products. Clinical relevant studies on these three modes are currently limited; domestic studies have shown that the PP+HDF group showed more significant coagulation function changes compared to using either alone; however, there was no significant difference in 3- and 6-month survival rates between the PP+HDF group and any single treatment, but both showed improvements.
1.4 MARS System
The MARS system is an external artificial liver support system established based on albumin dialysis, mainly consisting of blood circuits, albumin circuits, and conventional dialysis fluid circuits. It is commonly used abroad for liver failure caused by various reasons, itching due to cholestasis, and drug/food poisoning. In related studies of liver failure, the MARS system can improve patients’ bilirubin, blood ammonia, lactate, vasoactive substances, oxidative stress mediators, and pro-inflammatory factors, improve complications such as hepatic encephalopathy and hepatorenal syndrome, and increase survival rates at 7 days and 14 days, with a trend of improvement in survival rates at 28 days and 180 days but without significant statistical differences. A 2022 multicenter study on acute liver failure in the United States found that the 21-day survival rate without transplantation in patients treated with MARS significantly improved. Another meta-analysis also suggested that the frequency of MARS treatment is an independent predictor of survival in patients with acute-on-chronic liver failure (ACLF). Therefore, the impact of MARS on survival prognosis in liver failure patients remains somewhat controversial. MARS can provide long-term relief from itching for patients with cholestatic pruritus, with a median time of symptom relief lasting up to 3 months.
1.5 Prometheus System
The Prometheus system consists of a graded plasma separation and adsorption combined with a high-flux blood dialysis system, primarily aimed at clearing protein-bound toxins and water-soluble toxins, potentially outperforming the MARS system. The Prometheus system can effectively reduce biochemical indicators in patients with acute liver failure, facilitating their successful transition to liver transplantation. However, the survival benefits of the Prometheus system for ACLF patients are also controversial. In the HELIOS trial in Europe, there were no significant changes in 28-day and 90-day survival rates for ACLF patients treated with the Prometheus system, but significant survival benefits were noted in subgroups of patients with hepatorenal syndrome type I or MELD scores >30. Studies on ACLF combined with hepatorenal syndrome found that treatment with the Prometheus system could lower levels of asymmetric dimethylarginine (a nitric oxide synthase inhibitor) in plasma. Treatment with the Prometheus system can alleviate persistent pruritus symptoms in patients, with the longest symptom relief time lasting up to 4 weeks. After food or drug poisoning, the application of the Prometheus system can reduce liver injury and avoid the need for liver transplantation.
1.6 New Modes Registered for Clinical Trials
Currently, a new combined NBAL treatment registered at international clinical trial registries is the CABA system (BS330 plasma bilirubin adsorption + CA280 cytokine adsorption column) combined with PE, primarily aimed at treating end-stage liver disease patients, intending to reduce pro-inflammatory factors in these patients and improve their immune dysregulation state, but no related clinical results are available yet (NCT05780190).

2Combined BAL
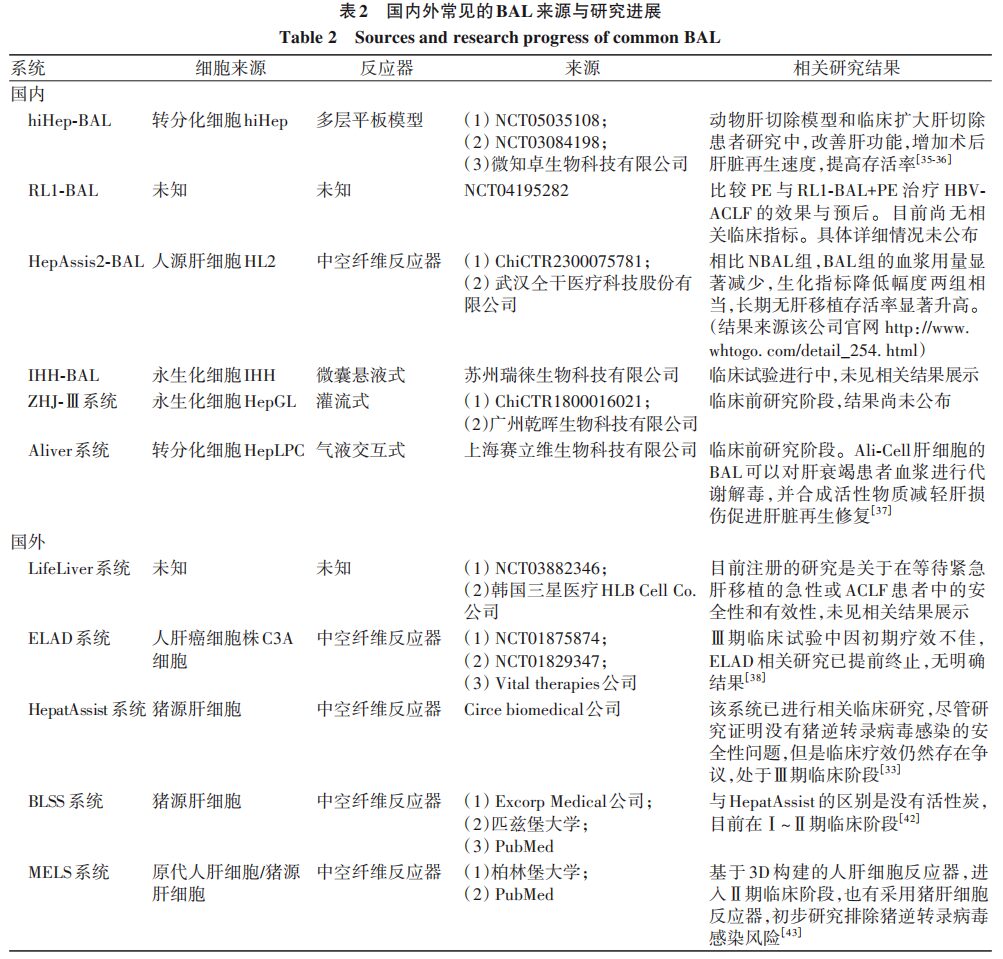
Combined BAL mainly consists of bioreactors, NBAL systems, and auxiliary circulation systems. Typically, blood is first cleared of toxic substances by NBAL, and then material exchange is performed by liver cells in BAL, closely resembling the functions of the human liver. To achieve optimal BAL effects, the liver cell sources in the bioreactor need to meet characteristics such as easy cultivation, long survival time, non-toxic side effects, and good physiological functions. Currently, the main research focuses on BAL cells include xenogeneic liver cells, primary human liver cells, tumor-derived liver cells, stem cells, and mesenchymal stem cells. The reactor must meet the structural requirements suitable for liver cell growth and high tissue compatibility, mainly seen in four structures: hollow fiber, perfusion type, plate type, and microencapsulated suspension. Due to the special requirements for reactors and liver cell sources, there is currently no widely applicable BAL mode in clinical settings. Here, we briefly summarize the research progress of combined BAL both domestically and internationally.
2.1 Research Progress on Combined BAL in China
2.1.1 Early Domestic BAL Research
Early researchers in China applied pig liver cells in bioreactors combined with PE to construct combined BAL for the treatment of patients with chronic severe viral hepatitis, finding that this treatment could continuously reduce levels of endotoxin, TNF-α, and IL-6, improving symptoms such as fatigue and abdominal distension. Although basic research and a small number of clinical studies indicated that pig liver cell-based BAL is safe and more effective than single NBAL treatment in severe liver disease patients, the potential infection risk due to the transfer of porcine endogenous retrovirus to the bioreactor’s filtration membrane has led to its prohibition in European countries. In 2014, Chinese researchers applied human liver cells (CL-1) in BAL combined with HP treatment in animal models of acute liver failure induced by D-galactosamine, resulting in significantly higher survival rates in treated animals compared to the control group, along with reduced ALT, TBil, and total bile acid levels. However, there are currently no clinical studies on CL-1 cell-based combined BAL.
2.1.2 Recent Domestic BAL Research
(1) hiHep-BAL: In 2023, Professor Huilijian’s research group transformed human umbilical cord fibroblasts into proliferative functional liver cells (hiHep) using liver transcription factors, constructing a new type of BAL. The hiHep-BAL has been shown to significantly reduce blood ammonia levels and improve coagulation function in animal liver resection models, as well as increase the regeneration speed after liver resection, improving survival rates. No related adverse reactions were observed in clinical treatments for patients undergoing extended liver resections, with decreased liver enzymes, blood ammonia, and inflammatory indicators after treatment, and increased average liver remnant volume on the 7th day post-treatment compared to untreated groups. Currently, hiHep-BAL has been registered for clinical trials to evaluate its efficacy in post-operative liver failure and acute liver failure. (2) Stem Cell-Based BAL: Professor Gao Yi confirmed that mesenchymal stem cells are a type of pluripotent stem cell that can inhibit liver injury caused by excessive inflammation and exert immune regulation and tissue regeneration effects through cytokine secretion and exosome release. Stem cell-based BAL has currently progressed to the clinical trial stage, but related research details have not yet been published. (3) Gas-liquid interactive biological artificial liver (Ali-BAL): This model uses transdifferentiated HepLPC as liver cells. Animal studies on drug-induced acute liver failure indicated that treatment with Ali-BAL for 3 hours significantly improved blood ammonia concentrations, biochemical and coagulation indexes, preventing the occurrence of hepatic encephalopathy, making it a potential treatment avenue for acute liver failure patients. (4) HepAssis2® System: This system uses human-derived liver cells HL2 to form BAL and is currently registered for clinical research at Zhongnan Hospital. Data published on its official website shows that compared to the NBAL group, the BAL group significantly reduced plasma usage, with biochemical indicators decreasing comparably in both groups, and significantly increased long-term survival rates without liver transplantation. (5) Other relevant BAL systems: The ZHJ-Ⅲ (immortalized HepGL cells) system and the RL-1 new BAL system are undergoing clinical research, with results yet to be published.
2.2 Common BAL Systems Abroad
2.2.1 ELAD System
The ELAD system is based on hollow fiber reactors loaded with liver tumor-derived cell line C3A cells, which effectively improve albumin synthesis and remove toxic substances from the patient by separating plasma through a venous-venous blood filter machine. However, clinical phase III trial results indicated that the treatment was terminated due to poor efficacy.
2.2.2 SRBAL System
In acute liver failure animal models, BAL with liver cell spheroid bioreactors effectively cleared serum ammonia, preserved renal function, and delayed increased intracranial pressure, improving survival rates. In patients with acute liver failure after the resection of 85% of the liver, SRBAL treatment improved the survival rates of these patients and lowered blood ammonia levels while accelerating liver regeneration. In non-human primate acute liver failure models, continuous treatment with SRBAL for 6 hours reduced blood ammonia and TBil levels, increased albumin levels, and improved survival rates without porcine endogenous retrovirus infections. In surviving cases, the proportion of necrotic and apoptotic liver cells in the SRBAL treatment group was lower, with reduced cytokines TNF-α, IL-8, IL-2β, and IFN-γ.
2.2.3 HepatAssist System and BLSS System
In studies of explosive liver failure and primary non-function patients after liver transplantation, there was no significant difference in adverse event rates between the HepatAssist group and the control group, and at the end of the study, all presented negative results for porcine endogenous retrovirus infections. There was no significant statistical difference in overall survival rates between the two groups, but there was a trend of increased survival rates in the HepatAssist treatment group for explosive liver failure patients compared to the control group. Due to controversies regarding the efficacy of HepatAssist in clinical studies, it is still in phase III clinical trials. The BLSS system lacks the activated carbon adsorption structure compared to the HepatAssist system, and current clinical relevant studies only suggest that there is no clear evidence of porcine endogenous retrovirus infections after standard treatment through nucleic acid and genomic screening, with follow-up trial progress yet to be disclosed.
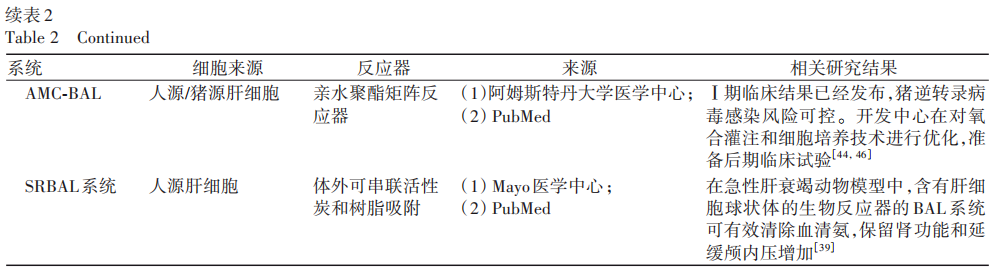
2.2.4 MELS System and AMC-BAL
MELS is tailored to the actual clinical needs of each patient, containing a multi-chamber bioreactor for external liver support treatment. The unit also includes a detoxification module capable of clearing albumin-bound toxins, and in cases of liver and renal failure, a dialysis module for continuous venous-venous blood filtration can be added. Studies comparing the MELS and AMC-BAL external devices showed that liver cells maintained physiological function for 7 days in both bioreactors, with an average decline of 9.7%. Among them, AMC-BAL exhibited higher lidocaine elimination on days 1 and 2, and higher ammonia elimination on day 7; the lactate dehydrogenase release from the MELS cell module significantly decreased on day 7. Early AMC-BAL-related experiments suggested that it could improve liver function, but the maintenance time was short. Therefore, researchers have upregulated liver cell mitochondrial function in the AMC-BAL system through high oxygenation, moderate perfusion, and 3D structure to enhance liver cell line functionality. A summary of common BAL-related characteristics both domestically and internationally is shown in Table 2.

3Summary and Outlook
Due to breakthroughs in “membrane technology,” various modes of NBAL have emerged based on the direction of toxin clearance in the body. The combination modes based on PE can supplement the loss of plasma coagulation factors, albumin, etc., caused by other modes, while other modes reduce the demand for blood products, increase the range and degree of toxin clearance in the body, clear inflammatory factors, provide a microenvironment for liver cell regeneration and repair, and block further disease progression. The design concept of mixed BAL is to combine the detoxification function of NBAL with the synthetic, transformation, and metabolic functions of BAL to provide external support for patients with liver failure, alleviate the burden on the damaged liver, and provide an environment and conditions for the regeneration and repair of the damaged liver. However, the two core challenges for BAL development are meeting the treatment conditions for liver cell sources and bioreactors. In recent years, breakthroughs in liver cell sourcing have been continuously achieved, and domestic scholars have made progress in the research direction of transdifferentiated cells and mesenchymal liver cells, providing new directions for BAL treatment. When stable and efficient BAL is widely applied in clinical treatment, the combined BAL system, integrating the detoxification function of NBAL, is likely to become the most effective external support treatment for patients with acute and chronic liver failure, effectively alleviating and improving the immune status and inflammatory response in critically ill patients, thus becoming a necessary means for critical support treatment in internal medicine.

https://www.lcgdbzz.org/cn/article/doi/10.12449/JCH240203

Huang Yue, Peng Hong, Luo Xinhua. Research Progress on Combined Artificial Liver. Clinical Journal of Hepatology and Biliary Diseases, 2024, 40(2): 233-238


The first release online|The “Clinical Journal of Hepatology and Biliary Diseases” 2024 Issue 2 “Clinical Practice and Research Progress of Artificial Liver Treatment” Special Issue (Executive Editor: Han Tao)

Review|Han Tao: Clinical Practice and Research Progress of Artificial Liver Treatment

Expert Forum|Zhou Xinmin: Artificial Liver Treatment in Early Stages of Liver Failure

Key Topics and Executive Editors of the “Clinical Journal of Hepatology and Biliary Diseases” 2024 Issues 1-10

■ The first professional journal for hepatobiliary diseases in China
■ Included in 13 major international databases such as Embase and Scopus
■ One of the top 100 academic journals in China
■ A fine science and technology journal in China
■ A top journal among the top 100 universities in China
■ Included in the “World Influence Index Report” for scientific journals
■ A core journal in Chinese core journals by Peking University Library
■ A statistical source journal for Chinese scientific papers
■ RCCSE Chinese core academic journal
Official Website:lcgdbzz.org
Official WeChat:lcgdbzz1985
Submission Inquiry:0431-88782044
Review Inquiry:0431-88783542
Reviewer Application Portal:Click to view
