
Acute myeloid leukemia (AML) is a malignant clonal disease of hematopoietic stem/progenitor cells. Approximately 20% of AML patients have mutations in isocitrate dehydrogenase 1 and 2 (IDH1/2), which lead to DNA hypermethylation and epigenetic abnormalities, thereby inhibiting hematopoietic cell differentiation, making it an early event in AML. However, the prognostic value of IDH mutations in adult patients with normal karyotype AML (NK-AML) remains controversial, and differences in induction treatment regimens may be one reason for this discrepancy.
Previously, daunorubicin + cytarabine (DA) was the standard induction therapy for AML patients. Clinical trials, including PALG, support the combination of cladribine (DAC) with DA to further improve induction therapy. Given the characteristics of IDH1/2 mutations, such as deep metabolic and epigenetic events, and the demethylation activity of cladribine, it is hypothesized that leukemia progenitor cells exhibit differences in clinical activity between the DA and DAC induction regimens. Therefore, this study aims to confirm that DAC induction therapy is significantly associated with improved outcomes in IDH2+ NK-AML patients.
2.1 Prognostic Value of IDH1/2 Mutations in Overall NK-AML Population and Different NPM1/FLT3 Mutation Status Subgroups
This study included 398 newly diagnosed NK-AML patients, of which 80 (20.1%) had IDH1/2 mutations (IDH1/2+). The overall complete remission rate (CR) was 75.4% (300/398); univariate and multivariate analyses showed that IDH1 and IDH2 gene mutations did not affect the complete remission rate. Stratifying the overall NK-AML population based on IDH1 mutation status revealed that IDH1+ patients had a declining trend in overall survival (OS), but no statistically significant difference; in contrast, univariate and multivariate analyses after excluding allogeneic hematopoietic stem cell transplantation (alloHSCT) showed that IDH2+ patients significantly benefited in terms of OS (Figure 1-A).
Subsequent analyses compared low-risk (LR: NPM1+/FLT3-ITD–) and high-risk (HR: FLT3-ITD+ , NPM1–/FLT3-ITD–) genotypic populations to determine the impact of NPM1/FLT3 genotype on the prognostic value of IDH1/2. Univariate analyses showed that IDH1 had an adverse impact on HR NK-AML patients’ OS (IDH1+ vs IDH1− OS rates were 15% vs 36%; P=0.03; Figure 1-B), and the impact was particularly significant for NPM1–/FLT3-ITD– (IDH1+ vs IDH1− OS rates were 15% vs 43%; P=0.026); multivariate analysis after excluding alloHSCT showed that IDH1 mutations were independently associated with increased mortality risk in HR NK-AML and NPM1–/FLT3-ITD– subgroup patients. Unlike IDH1, when assessed alongside NPM1 and FLT3 mutations, IDH2 mutations had a positive impact on the prognosis of both HR and LR subgroups (Figure 1-B,C); moreover, IDH2 R140 and IDH2 R172 mutations did not affect the survival of NPM1–/FLT3-ITD– subgroup. Notably, except for the NPM1−/FLT3-ITD− genotype, all other IDH2 R140 mutations were usually accompanied by NPM1 mutations (with or without FLT3-ITD); thus, the OS benefit for IDH2 mutation patients in the overall cohort was primarily driven by the NPM1+/IDH2-R140+ genotypic subgroup.
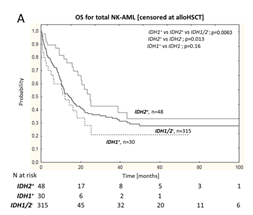
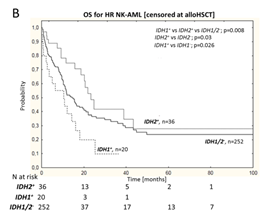
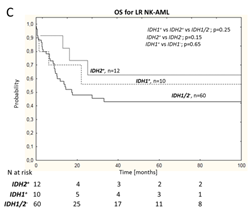
Figure 1 Overall NK-AML Population (A), HR NK-AML(B) and LR NK-AML(C) Patients’ OS
2.2 Prognostic Significance of IDH2 Mutations in Patients Receiving DA vs DAC Treatment
IDH2 mutations positively impacted the survival rate of NK AML patients receiving DAC treatment (IDH2+ vs IDH2-: 54% vs 33%, P=0.0087; Figure 2-A), but had no effect on the survival rate of patients receiving DA treatment (IDH2+ vs IDH2-: 21% vs 23%, P=0.22; Figure 2-B). In the DA treatment group, IDH2 R140 and IDH2 R172 mutations had no effect on OS.
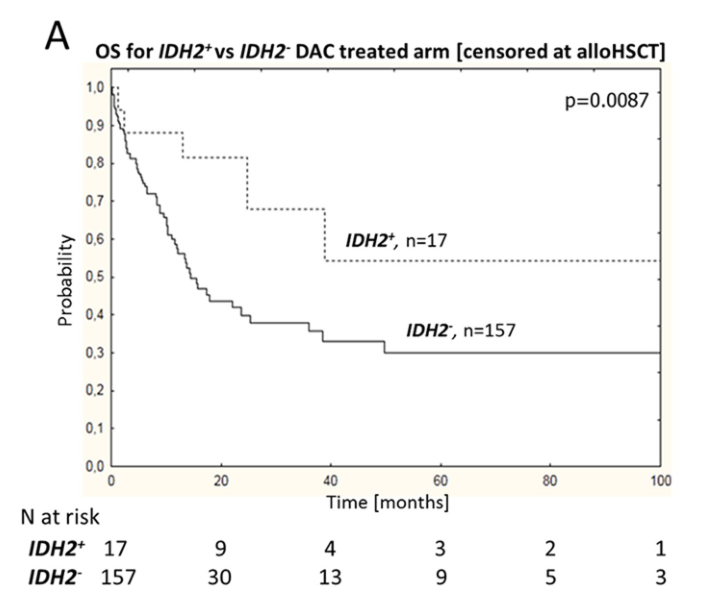
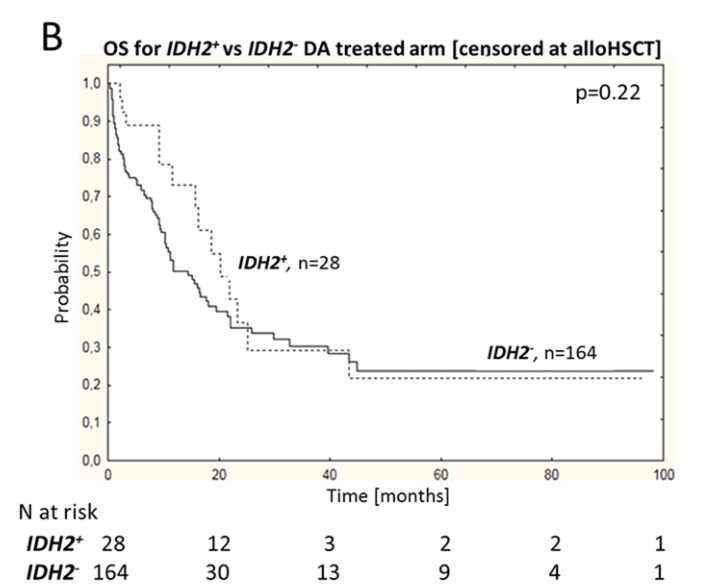
Figure 2 Impact of IDH2 Mutation Status on OS in DAC and DA Treatment Subgroups
2.3 Impact of Cladribine Combined with Standard DA Induction Therapy on Prognosis of IDH1/2+ NK-AML Patients
Further comparisons of clinical outcomes in IDH1/2+ patients receiving DAC vs DA treatment show that, after excluding HSCT, DAC induction therapy was associated with improved 4-year OS in high-risk IDH2+ patients compared to standard DA regimen (OS: 50% vs 13%; P=0.04; Figure 3-A/B); among which, the improvement in OS was particularly significant in the NPM1−/FLT3-ITD− subgroup (HR: 0.3; 95%CI 0.08-0.95; P=0.04); however, IDH2- or IDH1 patients’ OS did not improve. In the IDH2 + subgroup, the good benefit of cladribine was limited to younger patients (< 50 years). However, multivariate analysis for IDH2 + patients showed that after excluding alloHSCT, DAC induction therapy was independently associated with reduced mortality risk (HR: 0.21; 95%CI 0.056 – 0.8; P = 0.02).
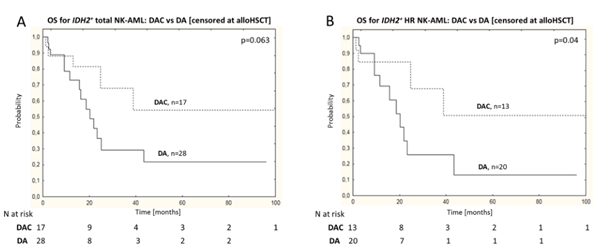
Figure 3: DAC vs DA Treatment of IDH2+ Overall NK-AML Patients (A) and HR NK-AML Patients (B) OS
IDH2 mutations induce the production of enzymes with novel variant activity that generate 2-hydroxyglutarate (2HG), which is a competitive inhibitor of 2-ketoglutarate-dependent enzymes, such as TET2 (an enzyme involved in DNA demethylation). Therefore, this study aims to evaluate whether cladribine can inhibit 2HG-dependent DNA hypermethylation in AML cells. HEL and MOLM14 cell lines were treated with synthetic cell-permeable derivatives of 2HG and octyl-2HG alone or in combination with cladribine for 24 hours, and the results showed that only the octyl-2HG treatment group exhibited significantly increased DNA methylation levels.
Furthermore, compared to IDH2 wild-type (IDH2wt) cells, IDH2 R140 and IDH2 R172 mutant cells induced DNA hypermethylation (Figure 4). Compared to cells treated with the specific IDH2-R140 inhibitor AGI-6780, cells treated with cladribine (10 nM or 25 nM, 24 h) showed reduced levels of 5-methylcytosine, and cladribine combined with AGI-6780 further reduced DNA methylation. Further studies found that cladribine inhibited S-adenosylhomocysteine hydrolase, which is involved in the biosynthesis of S-adenosylmethionine (SAM), and can serve as a methyl donor in DNA methylation reactions; thus, cladribine primarily inhibits DNA methylation by affecting SAM levels.
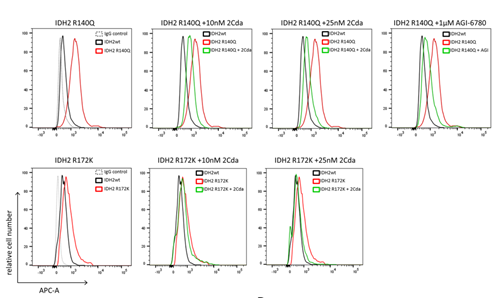
Figure 4: Cladribine Inhibits IDH2 R140Q and R172K Mutation-Induced DNA Hypermethylation

The results indicate that combining cladribine with standard AML induction therapy can improve the prognosis of patients with IDH2 mutations. Given that IDH2 mutations can induce DNA hypermethylation, the combination of cladribine with DA may have a synergistic effect related to the demethylation activity of cladribine. The value of cladribine treatment for IDH1/2+ patients should be further evaluated, including its effects on complete remission rates, overall survival, and residual disease impact.

Libura M,et al.Sci Rep. 2021 May 11;11(1):10017.

Welcome to share
This article is translated by the oncology team of the Ministry of Health and is intended for medical professionals only! Welcome to share!
