From May 30 to June 3, 2025, the largest global oncology conference, the American Society of Clinical Oncology (ASCO) Annual Meeting, was held in Chicago, USA. Researchers including Philippe Armand presented data from the waveLINE-003 Phase II study, exploring the recommended treatment dose, safety, and efficacy of the novel antibody-drug conjugate (ADC) Zilovertamab vedotin (ZV) for patients with relapsed/refractory (R/R) diffuse large B-cell lymphoma (DLBCL). The summary is as follows:

1
Research Background and Objectives
“
The prognosis for R/R DLBCL patients is generally poor. Zilovertamab vedotin (ZV) is a novel ADC targeting receptor tyrosine kinase-like orphan receptor 1 (ROR1), which has shown good efficacy in DLBCL patients. Here, the researchers report the results of the dose-confirmation part of the waveLINE-003 study (NCT05139017), which evaluated the efficacy of ZV combined with rituximab + gemcitabine + oxaliplatin (R-GemOx) regimen for R/R DLBCL patients.

2
Research Methods
“
waveLINE-003 is a Phase II/III trial that included adult patients with R/R DLBCL. These patients had previously received ≥1 line of treatment and did not meet the criteria for chimeric antigen receptor (CAR) T-cell therapy or autologous stem cell transplantation (ASCT) or had failed such treatments (Cohort A). In the dose-confirmation phase, patients received ZV (1.5, 1.75, or 2.0 mg/kg) + R-GemOx treatment every 3 weeks (Q3W) for ≥6 cycles.
The primary endpoint of the Phase II stage was safety and the recommended treatment dose (RP2D) in the Phase II stage. Secondary endpoints included the objective response rate (ORR), duration of response (DOR), and overall survival (OS) assessed by central review according to the Lugano 2014 efficacy evaluation criteria.

3
Research Results
“
The data cutoff date was August 1, 2024, and a total of 40 patients were included in the study and treated with R-GemOx + different doses of ZV: ZV 1.5 mg/kg group (n=17), ZV 1.75 mg/kg group (n=16), or ZV 2.0 mg/kg group (n=7); among them, 22 patients (55%) were aged ≥65 years, and 8 patients (20%) relapsed after >12 months. The median number of prior treatment lines was 2.0, with 7 patients (18%) having previously received CAR-T cell therapy and 7 patients (18%) having previously undergone ASCT. The median follow-up time was 9.8 months.
A total of 7 patients reported dose-limiting toxicities (DLT) (1 case in the ZV 1.5 mg/kg group, 2 cases in the ZV 1.75 mg/kg group, and 4 cases in the ZV 2.0 mg/kg group). 39 patients (98%) reported treatment-related adverse events (TRAE); the most common were diarrhea (n=18 [45%]), nausea (n=15 [38%]), anemia (n=11 [28%]), and thrombocytopenia (n=11 [28%]).
26 patients (65%) reported ≥ grade 3 treatment-related AEs, with the most common being neutropenia (n=9 [23%]), decreased neutrophil count (n=9 [23%]), thrombocytopenia (n=9 [23%]), and anemia (n=8 [20%]). 2 patients discontinued due to TRAE (sepsis and respiratory failure), and 1 patient died from treatment-related sepsis, all occurring in the 2.0 mg/kg dose group. The determined RP2D was 1.75 mg/kg.
The ORR for each group was: ZV 1.5 mg/kg group 27% (3 cases of complete response [CR], 1 case of partial response [PR]), ZV 1.75 mg/kg group 56% (8 cases of CR, 1 case of PR), ZV 2.0 mg/kg group 57% (3 cases of CR, 1 case of PR) (see Figure 1).
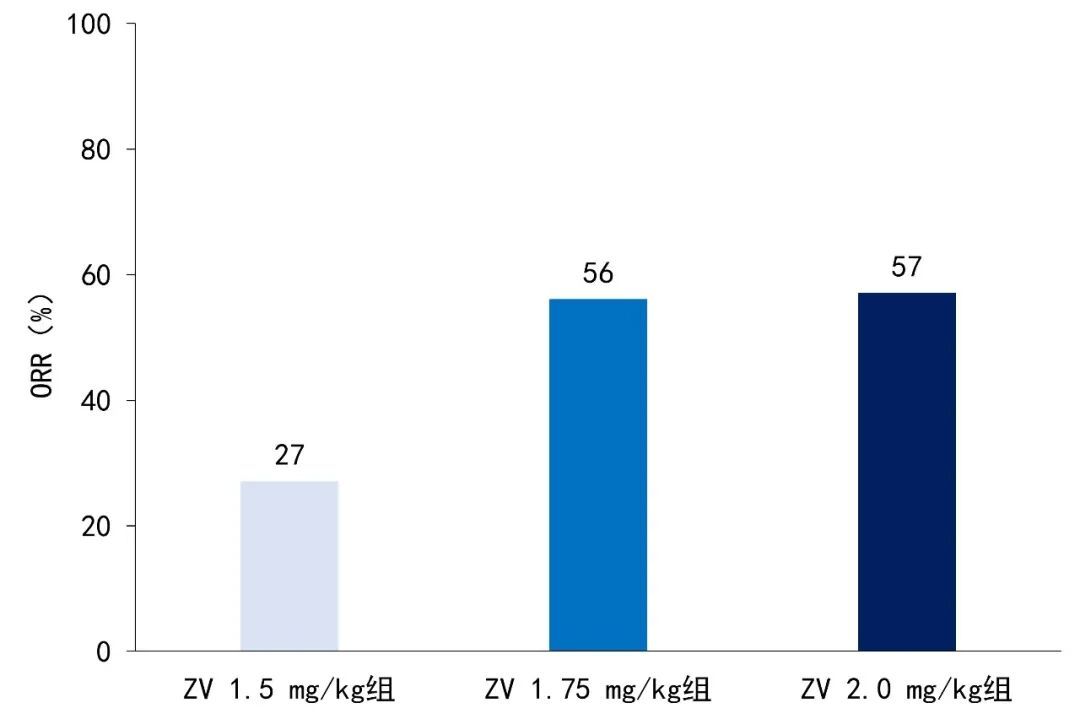
Figure 1. ORR of patients in different ZV dose groups
The median DORs were 14.4 months, 8.7 months, and not reached (NR). The median OS were 11.5 months, not reached, and 7.4 months, with 6-month OS rates of 70.0%, 78.8%, and 68.6% respectively (see Figure 2).
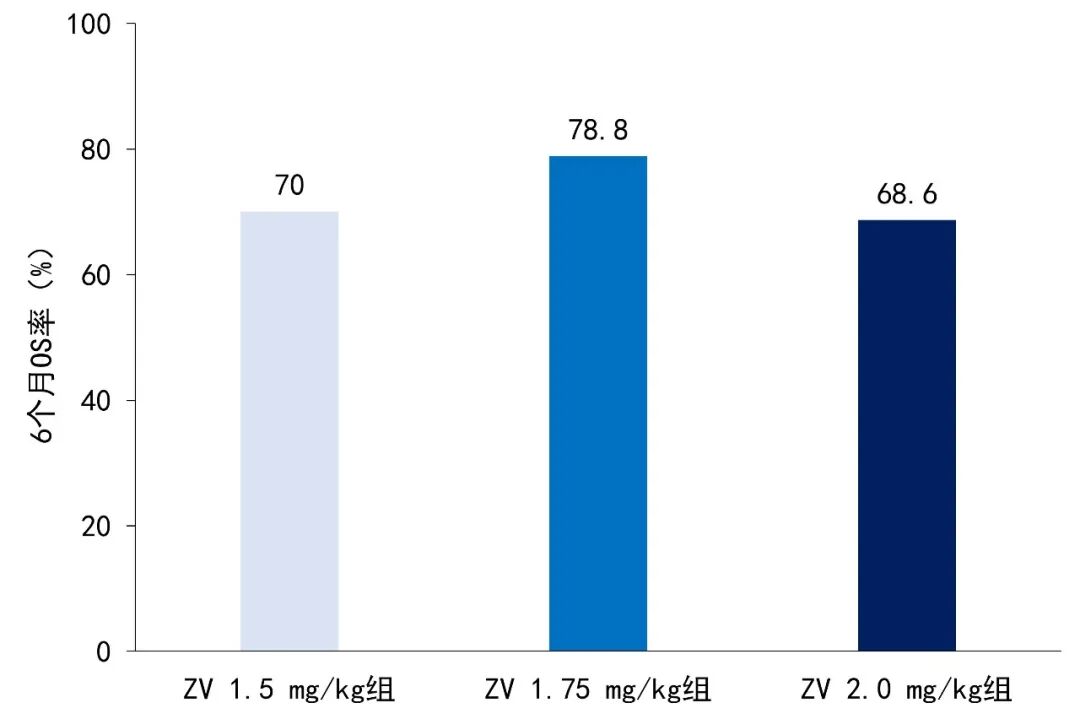
Figure 2. 6-month OS rates of patients in different ZV dose groups

4
Research Conclusions
“
At the RP2D (ZV 1.75 mg/kg), the ZV + R-GemOx regimen demonstrated good efficacy and acceptable safety in R/R DLBCL patients. The study is currently in the Phase III stage, randomly assigning patients to the ZV-RGemOx group or the RGemOx group for treatment. Clinical trial information: NCT05139017.
References:
1. Philippe Armand et al. WaveLINE-003: Phase 2/3 trial of zilovertamab vedotin plus standard of care in relapsed/refractory diffuse large B-cell lymphoma.. JCO 43, 7005-7005(2025).
Editor: Kitty
Reviewer: Andy
Typesetting: Kitty
Disclaimer: Some of the images and text materials in this article are sourced from the internet, aimed at disseminating medical science information and cutting-edge progress. If there is any infringement, please contact the platform for deletion; the content is for reference and learning purposes for medical professionals and the general public, and does not constitute professional medical advice or diagnosis. If you have any health concerns or symptoms, please seek medical attention promptly.
