
At the recent 2025 European Hematology Association (EHA) Annual Meeting and the International Conference on Malignant Lymphoma (ICML), not only were continuous breakthroughs in the field of lymphoma treatment witnessed, but a series of studies also validated the immense potential of precision immunotherapy in enhancing patient survival benefits. “Blood News” focuses on this international event, meticulously organizing the research context of innovative drugs: from frontline treatment optimization to breakthroughs in innovative solutions for relapsed/refractory patients; from personalized treatment explorations for elderly and frail populations to innovative practices in precision medication. This issue will systematically present the clinical value of these research findings, analyze the development direction of lymphoma diagnosis and treatment in the era of immunotherapy, and provide clinical practitioners with academic references that are both cutting-edge and practical.

Aggressive Lymphoma
Pola Expands Frontline Treatment Boundaries
In low-risk populations, the application of Pola-R-CHP achieved a remission rate exceeding 80%, and the “Pola+” regimen realized deep remission and long-term benefits.

Key Points Overview
Multiple studies indicate that the Pola-R-CHP regimen is also safe and effective as a frontline treatment for special patients such as low-risk, elderly, and those with comorbidities (HLH), particularly in low-risk populations where the CRR exceeds 80%;
The POLARIX study’s Asian subgroup 5-year follow-up data shows that the Pola-R-CHP regimen significantly improves long-term prognosis, with a 5-year PFS rate rising to 64%, OS rate reaching 84.6%, and a significant reduction in the risk of disease progression or death, solidifying its position as the first-line standard treatment for Asian DLBCL;
The phase II study of Glofitumab combined with Pola-R-CHP as first-line treatment for DLBCL patients achieved its primary endpoint, with an ORR of 95% and CRR of 80% at EOT, and a 1-year PFS of 95%, with no new safety signals detected;
The R-Pola-Glo trial showed an ORR of 90% at EOT, with a CMR of 82%, and approximately half of the patients transitioned from PR to CMR during subsequent consolidation therapy, demonstrating excellent deep remission capabilities, and is expected to become a first-line choice for newly diagnosed aggressive lymphoma patients unsuitable for adequate R-CHOP;
Diffuse large B-cell lymphoma (DLBCL), as the most common subtype of aggressive non-Hodgkin lymphoma, has always been the focus of clinical research for optimizing treatment strategies. Based on the results of the POLARIX trial, the Pola-R-CHP regimen has become the current first-line standard treatment (SOC) for DLBCL, significantly improving the progression-free survival (PFS) of many patients. At both meetings, multiple studies focusing on Pola-R-CHP (including Polatuzumab vedotin, Rituximab, Cyclophosphamide, Doxorubicin, and Prednisone) and clinical research covering the Pola regimen were prominently presented, further highlighting its clinical value and benefit advantages from multiple dimensions, providing key support for the innovation and promotion of first-line treatment for DLBCL.
Pola-R-CHP – Significant Efficacy Benefits Across Different Populations, 5-Year PFS Up to 64%
At the ICML conference, the POLARIX study’s Asian subgroup 5-year follow-up data was released (288), with a median follow-up of 60 months, showing that the Pola-R-CHP regimen significantly improves long-term prognosis compared to the R-CHOP regimen, with a 5-year progression-free survival (PFS) rate rising to 64% (vs 57.3%, HR 0.74), overall survival (OS) rate reaching 84.6% (vs 77.7%, HR 0.65), and a significant reduction in the risk of disease progression or death, fully validating the continuous benefit of the Pola-R-CHP regimen as the first-line standard treatment for Asian DLBCL, providing critical evidence support for solidifying its clinical position.
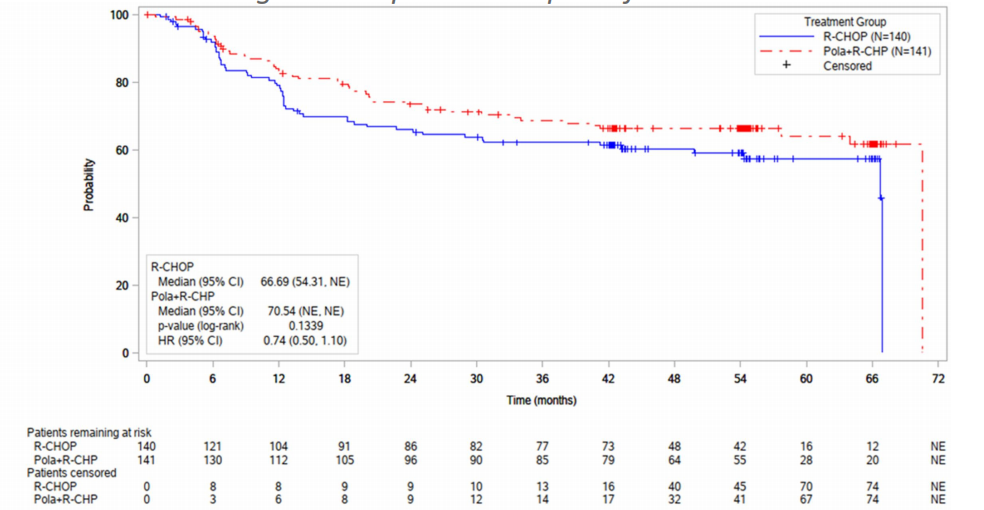
Kaplan-Meier survival curve of PFS in the POLARIX study
Additionally, both meetings reported data on Glofitumab and other CD20/CD3 bispecific antibodies combined with Pola-R-CHP (74, S247), where the multicenter phase II study of Glofitumab combined with Pola-R-CHP showed that it achieved the preset primary endpoint in newly diagnosed DLBCL patients: an overall response rate (ORR) of 95%, a complete response rate (CRR) of 80%, and a 1-year PFS of 95%, with no new safety signals detected, warranting further evaluation. This study validated the synergistic effect of bispecific antibodies with chemotherapy immunotherapy, demonstrating good efficacy and safety, providing strategic references for other lymphoma subtypes.
In special populations, two studies from China (PS1967, PF984) filled the evidence gap for the application of the Pola-R-CHP regimen in low-risk (IPI 0-1) or patients not meeting the POLARIX trial enrollment criteria, achieving extremely high remission rates (CRR > 80%) with controllable safety. PB3250 explored the value of a reduced-dose regimen (dr-Pola-R-CHP) in patients aged ≥70 years with a Charlson Comorbidity Index (CCI) ≥2: the overall ORR reached 100%, with a CRR of 75%, and patients with a relative dose intensity (RDI) >40% showed the best efficacy (CRR 85.7%). PB3323 preliminarily demonstrated the potential of Pola-R-CHP in treating hemophagocytic lymphohistiocytosis (HLH)-related DLBCL, effectively and rapidly controlling HLH-related symptoms, achieving high remission rates and good short-term survival outcomes, with good tolerability. The above results suggest that the Pola-R-CHP regimen is also safe and effective in special patients such as low-risk, elderly, and those with comorbidities.
Multiple real-world studies further corroborate its excellent clinical value. A large retrospective study in the United States included 535 patients treated with first-line Pola-R-CHP (PS1914), showing an ORR of up to 92%, a CRR of 80%, and 1-year PFS and OS rates of 81% and 91%, respectively; and the cell origin (GCB/non-GCB) did not affect survival benefits. Real-world data from China (PB3280), Spain (PB3252), and Kuwait (PB3294) also confirmed that this regimen demonstrates good efficacy and safety in clinical practice, with the potential to improve patient prognosis.
“Pola+” Innovative Regimen – Exceptional Deep Remission Capability
In addition to the Pola-R-CHP regimen, three prospective phase II studies focused on elderly frail DLBCL patients, verifying that the Pola-centered combination regimens also have high remission rates and controllable safety. In oral report S248, R-Pola-Glo demonstrated exceptional deep remission capabilities in newly diagnosed aggressive lymphoma patients unsuitable for adequate R-CHOP: an ORR of 90% at EOT, a complete metabolic response rate (CMR) of 82%, and approximately half of the patients transitioned from partial remission (PR) to CMR during subsequent consolidation therapy. Overall safety was controllable, with 53% of patients experiencing no ≥3 grade toxicity throughout the treatment process, making it a promising new first-line treatment option for this patient population. The other two studies (PF943, PF921) explored different pathways of combining Pola with BTK inhibitors and other drugs, further enriching the treatment spectrum in the field.
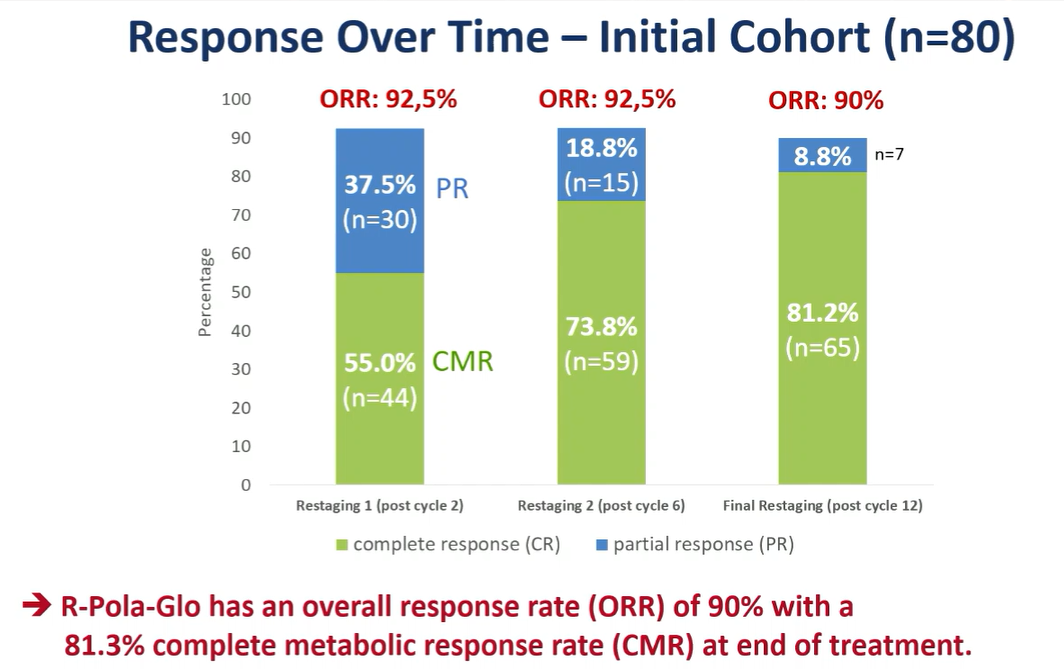
Effectiveness of different cycles in the R-Pola-Glo trial
The core breakthrough of the above “Pola+” studies lies in the clever combination of drugs with different mechanisms of action to form a Pola-centered combination regimen, achieving deep remission benefits far exceeding previous regimens in the elderly frail patient population, with the depth of remission expected to continue deepening as treatment progresses, while optimizing the safety profile, providing valuable individualized treatment options for clinicians.
In summary, the Pola-based regimen not only achieves efficacy enhancement but also reshapes the treatment paradigm for DLBCL. Especially in low-risk, elderly, and complex cases, it greatly expands the application boundaries through dose optimization and complementary mechanisms, bringing hope for the dawn of a new era of personalized treatment for DLBCL. Through the accumulation of multidimensional clinical trials and real-world data, it collectively depicts the future prospect of Pola transitioning from a standard treatment cornerstone to providing safe and effective treatment options for a broader patient population, highlighting its comprehensive advantages of “excellent efficacy, wide applicability, and controllable safety,” further reinforcing its position as a first-line preferred regimen.
New Breakthroughs in Relapsed/Refractory Lymphoma
The second-line Glofit-GemOx regimen achieved a 2-year OS rate of 59%, and the efficacy benefits of Pola-R-GemOx doubled.

Key Points Overview
The phase III STARGLO trial’s 2-year follow-up data shows that the fixed-duration Glofit-GemOx regimen has a median PFS of 13.8 months, with a CR rate soaring to 58.5%; and among patients who achieved CR at EOT, most maintained remission for 1 year post-EOT; in the 2L patient subgroup, the Glofit-GemOx regimen achieved an 18-month PFS rate of 52.2%, and a 24-month OS rate of 59.9% (vs 38.9%); supporting its early use as a preferred on-the-spot therapy for R/R DLBCL patients who do not meet transplant criteria;
The Pola-R-GemOx regimen extended the median OS to 19.5 months, reducing the risk of death by 40%, and the OS benefits were consistent across most subgroups, including ABC/GCB subtypes. The median PFS increased to 7.4 months, with CRR and ORR both doubling to 40.3% and 52.7%, respectively. This provides an efficient alternative treatment option for R/R DLBCL patients who have not received Pola and do not meet transplant criteria, while avoiding the risks of T-cell exhaustion regimens and their potential adverse effects on subsequent treatments (CAR-T);
In the treatment field of relapsed/refractory (R/R) DLBCL, research on chemotherapy-free strategies and innovative combination regimens is becoming a key direction to break through current treatment dilemmas, significantly reshaping existing treatment practice patterns. For example, treatment strategies based on Glofitumab have shown transformative application potential. The phase III STARGLO trial (PS1909, 76) 2-year follow-up data shows that the fixed-duration Glofit-GemOx regimen significantly extended the median OS of the overall population (NE vs 13.5 months), with a median PFS of 13.8 months (vs 3.6 months), and the CR rate increased from 25.3% to 58.5%; and among patients who achieved CR at EOT, the 1-year OS and PFS rates were as high as 89.3% and 82.4%, indicating that most patients maintained remission. Notably, in the second-line (2L) patient subgroup, the Glofit-GemOx regimen showed particularly impressive treatment benefits: an 18-month PFS rate of 52.2% (vs 28.3%), with significant separation of OS curves between the two groups, and a 24-month OS rate of 59.9% (vs 38.9%), with a positive trend of sustained benefits. The above data indicates that the Glofit-GemOx regimen can induce deep and early molecular remission, and continues to show more significant advantages in survival benefits and remission rates, supporting its early use as a preferred on-the-spot therapy for R/R DLBCL patients who do not meet transplant criteria.
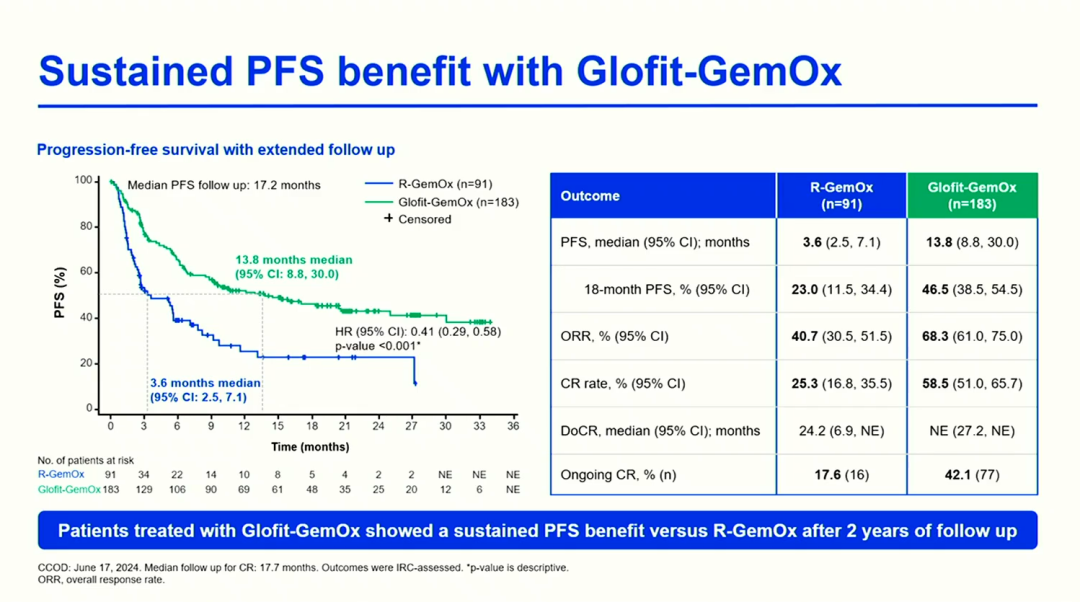
STARGLOTrialTotal PopulationPFSBenefitSituation
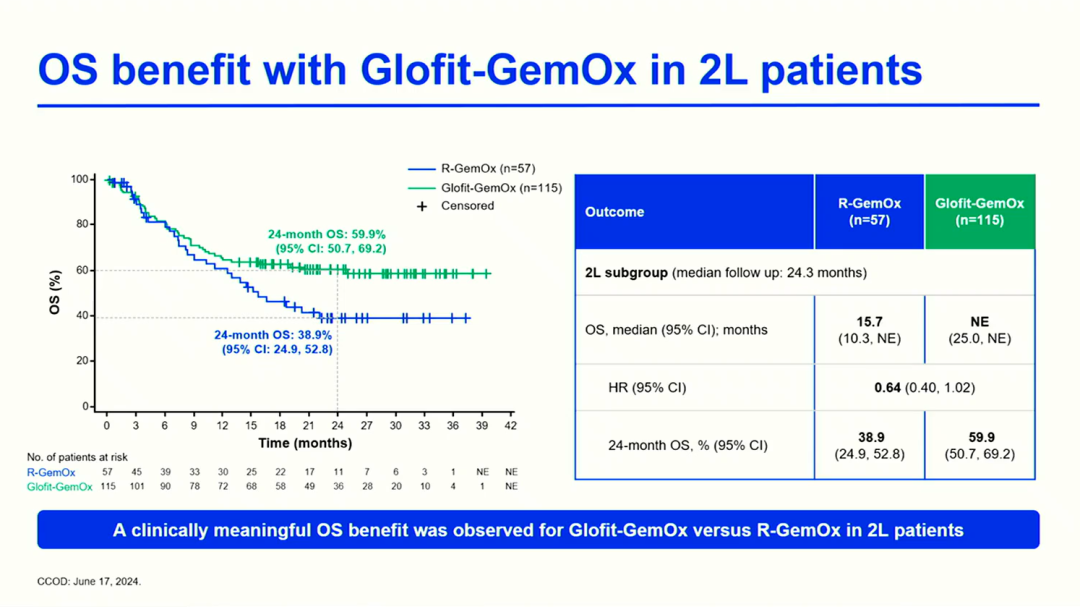
STARGLOTrialIn2LPatientSubgroupOSBenefitSituation
For patients with TP53 mutations or ≥2 lines of treatment failure, Glofitumab combined with BTK inhibitors, PD-1 monoclonal antibodies, and other chemotherapy-free regimens or salvage chemotherapy showed high efficacy and good tolerability (PB3271, PB3225, PS1963). The LOTIS-7 study (PS1911) innovatively explored the synergistic effect of Glofitumab combined with CD19 ADC: in heavily pretreated patients, the ORR reached 95.5%, and the CRR was 90.9%, while evidence of sustained T-cell activation and other synergistic mechanisms was also observed. Additionally, for patients with primary central nervous system lymphoma (R/R PCNSL), Glofitumab monotherapy (PF969) achieved an ORR of 87.5%, and 5 patients successfully bridged to CAR-T with sustained remission, providing new ideas for treating intracranial lesions.
The application of Pola is also expected to establish new treatment standards. The phase III POLARGO trial (S101, 7) confirmed that the Pola-R-GemOx regimen achieved a leap in survival compared to R-GemOx: the median OS extended from 12.5 months to 19.5 months, with a 40% reduction in the risk of death (HR=0.60, P=0.0017), and the OS benefits were consistent across most subgroups, including ABC/GCB molecular subtypes. The median PFS increased to 7.4 months (vs 2.7 months), and both CRR and ORR doubled to 40.3% and 52.7% (vs 19%, 24.6%). This regimen provides an efficient alternative treatment option for R/R DLBCL patients who have not received Pola and do not meet transplant criteria, while avoiding the risks of T-cell exhaustion regimens and their potential adverse effects on subsequent treatments. The Polish lymphoma research group (PF956) real-world study also verified that the Pola-RB regimen can serve as a feasible bridging treatment and subsequent treatment option for R/R DLBCL, especially in patients without large tumors and those sensitive to previous chemotherapy (ORR > 70%), but attention should be paid to the immunosuppressive effects of bendamustine, which may limit its applicability before CAR-T cell therapy.
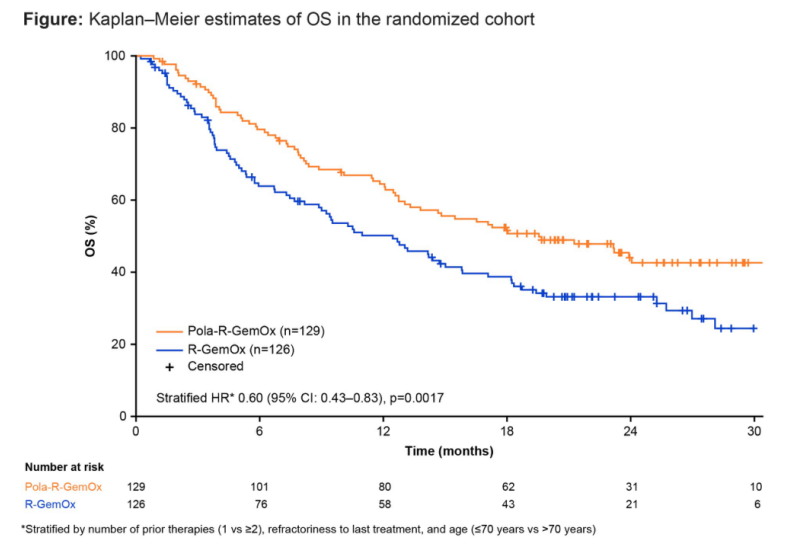
Kaplan-Meier survival curve of OS in the POLARGO trial random cohort
The clinical value of the above innovative regimens is reflected in three dimensions: first, survival benefits break through the limits of traditional regimens; second, fixed-duration or chemotherapy-free regimens are expected to shorten treatment cycles and optimize medical resource allocation; third, deep remission creates a window for subsequent treatment. These advances mark a fundamental shift in the treatment paradigm for R/R DLBCL, indicating that precision stratified treatment represented by chemotherapy-free regimens and innovative combination strategies will push clinical practice into a new era.

Indolent Lymphoma
Optimizing Comprehensive Management from Frontline to Relapse
Fixed-duration subcutaneous injection of Mosun is a safe and effective management choice for sustained remission.

Key Points Overview
The MITHIC-FL1 trial shows that first-line monotherapy with Mosun SC achieves a high CRR of 79%, with an ORR of 95%, and most FL patients maintain progression-free survival, with efficacy unaffected by clinical or cytogenetic risk factors;
The fixed-duration Mosun SC regimen demonstrates durable efficacy in R/R FL patients, with an overall CRR of 62%, and high-risk individuals still maintain significant efficacy; the median duration of complete response (DOCR) reaches 34.6 months, with an 18-month PFS of 57%, providing a new option for efficient outpatient management of patients who have failed traditional treatments. Long-term follow-up data confirms sustained benefits: with a median follow-up of 49.4 months, the median PFS for CR patients reaches 53.2 months, and the 42-month PFS rate is 64%;
The MORNINGSUN study shows that Mosun SC treatment achieves an ORR of 75% in MZL, with 61% of patients maintaining CMR; safety is manageable, reflecting the feasibility of outpatient chemotherapy-free regimens;
The M-Pola regimen achieves an overall ORR of 88%, with a CRR of 79%, TTR of 3 months, and median PFS and OS of 19 months and 21 months, respectively, making it a treatment option for R/R MCL patients to achieve rapid disease control and long-term survival benefits;
In the field of indolent lymphoma treatment, innovative drugs such as Obinutuzumab, Polatuzumab vedotin, Mosun, and Glofitumab continue to expand their clinical value, providing new treatment strategies for different subtypes of patients:
Follicular Lymphoma (FL): Optimizing Comprehensive Management from Frontline to Relapse
The extended follow-up data from the MITHIC-FL1 trial (27) shows that for newly diagnosed high tumor burden FL patients, monotherapy with subcutaneous Mosun (Mosun SC) achieves a high CRR of 79%, with an ORR of 95%, and most patients maintain progression-free survival, with efficacy unaffected by clinical or cytogenetic risk factors, estimating a 12-month PFS of 89%. Additionally, a real-world study from China (PF913) included 253 newly diagnosed FL patients, showing that first-line treatment with Obinutuzumab combined with chemotherapy achieved a best CMR of 73.0%, with an ORR of 99.6%, and in-depth analysis suggests that Obinutuzumab-based regimens better fit the characteristics of the lower FL International Prognostic Index (FLIPI) ratio in the Chinese population, supporting its use as a first-line treatment option for FL.
The fixed-duration Mosun SC regimen (PS1872) also demonstrates durable efficacy in R/R FL patients: with a median follow-up of 26.1 months, the overall CRR reaches 62%. Even high-risk individuals with Ann Arbor stage III/IV, bulky disease, and progression within 24 months (POD24) still maintain significant efficacy, with CRRs of 61%, 59%, and 56%, respectively. The median duration of complete response (DOCR) reaches 34.6 months, with an 18-month PFS of 57%, providing a new option for efficient outpatient management of patients who have failed traditional treatments. Long-term follow-up data (PF883) further verifies sustained benefits: with a median follow-up of 49.4 months, the median PFS for CR patients reaches 53.2 months, and the 42-month PFS rate is 64%; high-risk subgroups (POD24, double-refractory) still maintain DOCR rates of 68% and 63%, demonstrating its durable remission capability; infection events mostly occur early in treatment, with overall good safety, supporting the clinical application of fixed-duration regimens. Real-world applications (PB3329) also show that it bridges CR patients to autologous transplantation with PFS >24 months, highlighting its potential as a bridging treatment before salvage or transplantation.
Additionally, Mosun or Obinutuzumab combined with Lenalidomide as treatment regimens for R/R FL patients (PF1003, PS1907) show synergistic benefits, providing promising treatment options for patients with limited choices.
Marginal Zone Lymphoma (MZL) – Single Agent/Combination Approaches, Advancing First-Line Chemotherapy-Free Regimens
In the MZL field, the MORNINGSUN study of Mosun SC as first-line treatment achieved clinically significant breakthroughs (S232, 99): with a median follow-up of 11.3 months, the ORR reached 75%, with 61% of patients maintaining CMR. Safety is manageable, with injection site reactions being grade 1-2, and CRS being low-grade; 64% of patients successfully treated in community medical centers, marking the feasibility of outpatient chemotherapy-free regimens. The OLYMP-1 study conducted by the German Lymphoma Alliance (GLA) confirmed (PS1873) that Obinutuzumab monotherapy as first-line treatment for MZL shows significant efficacy and overall good tolerability: after 6 cycles of induction, the overall ORR reached 66%, with the best efficacy total ORR reaching 82%; the 2-year PFS and OS rates were 84% and 93%, respectively. Two other studies from China explored the rationale of combining Obinutuzumab with BTK inhibitors (PS1902, PS1905), and mechanistic studies also revealed that the O2 regimen could achieve synergistic anti-tumor effects by enhancing ADCC effects.
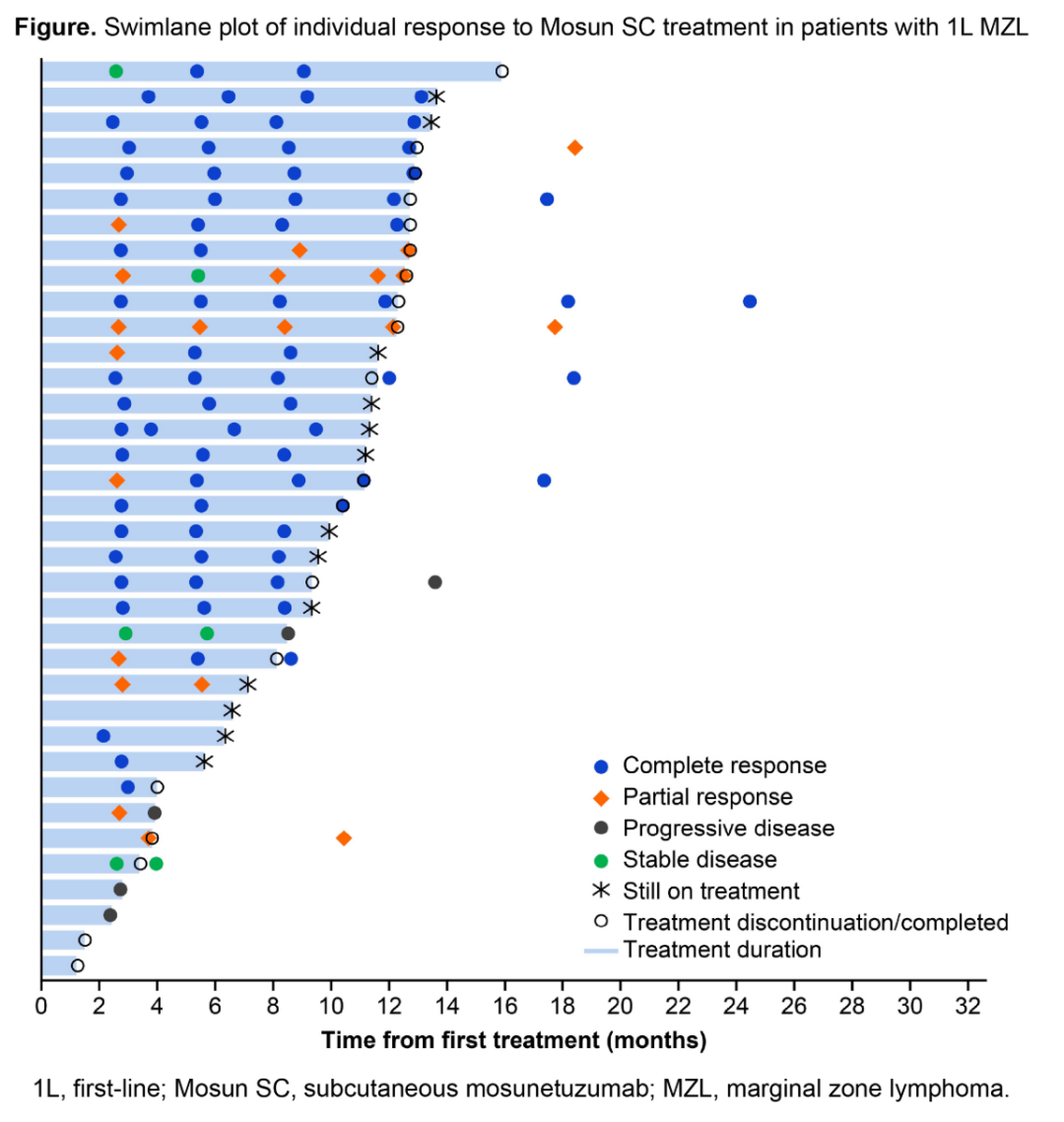
Swim lane diagram of MZL patients’ responses to first-line Mosun SC treatment
Mantle Cell Lymphoma (MCL) – Breaking Through the BTKi Resistance Dilemma
In R/R MCL patients treated with BTK inhibitors, the fixed-duration outpatient Mosun SC combined with Pola (M-Pola) phase II study (50) shows that this regimen has strong efficacy, including in high-risk subgroups and patients after CAR-T cell therapy: the overall ORR reached 88%, with a CRR of 79%, a median time to response (TTR) of 3 months, and median PFS and OS of 19 months and 21 months, respectively, making the M-Pola regimen a treatment option for R/R MCL patients needing rapid disease control and long-term survival benefits. For refractory MCL patients who relapse after cBTKi or CAR-T treatment, the GoldiLox study conducted by the Australian Leukemia and Lymphoma Group (ALLG) (PS1874) shows that Glofitumab combined with non-covalent BTK inhibitors demonstrates good efficacy – with an ORR of 77% and a CRR of 69%, and good tolerability achieved through enhanced steroid prevention strategies; at a median follow-up of 5.6 months, 81% of patients maintained remission, providing a new strategy for the deep response in the cBTKi resistant population. For high-risk R/R MCL with dual resistance to BTK/BCL-2 inhibitors, the Pola combined with Obinutuzumab ± chemotherapy salvage regimen shows application potential (PB3183), with 55.6% achieving PR, providing a transitional pathway for traditional treatment failures before CAR-T cell therapy.
These studies expand the application scenarios of innovative drugs in indolent lymphomas from multiple dimensions, including breaking through resistance bottlenecks, enhancing remission depth, exploring combination strategies for synergistic effects, and providing bridging strategies, offering more evidence support for clinical practice, especially in the management of refractory patients and the exploration of chemotherapy-free regimens. We look forward to larger sample studies of the above regimens to further validate their long-term efficacy and safety.
References: (Scroll up and down to see more)
1. 2025 ICML, YUQIN SONG, 288.
2. 2025 EHA. Ren Y. 06/14/2025; 4161041; PS1967
3. 2025 EHA. Ren Y. 06/13/2025; 4160390; PF984
4. 2025 EHA. Shah K. 06/12/2025; 4162323; PB3250
5. 2025 EHA. Magomedova A. 06/12/2025; 4162397; PB3324
6. 2025 EHA. Sun J. 06/12/2025; 4162396; PB3323
7. 2025 EHA. Thiruvengadam S. 06/14/2025; 4160988; PS1914
8. 2025 EHA. He J. 06/12/2025; 4162353; PB3280
9. 2025 EHA. Bobes A. 06/12/2025; 4162325; PB3252
10. 2025 EHA. Hamed R. 06/12/2025; 4162367; PB3294
11. 2025 EHA. Melchardt T. 06/15/2025; 4159325; S248
12. 2025 EHA. Ren Y. 06/13/2025; 4160349; PF943
13. 2025 EHA. Wang W. 06/13/2025; 4160327; PF921
14. 2025 EHA. Matasar M. 06/14/2025; 4159178; S101
15. 2025 EHA. Olszewska-Szopa M. 06/13/2025; 4160362; PF956
16. 2025 EHA. Gregory G. 06/14/2025; 4160983; PS1909
17. 2025 EHA. Zhao X. 06/12/2025; 4162344; PB3271
18. 2025 EHA. Zhou D. 06/12/2025; 4162298; PB3225
19. 2025 EHA. Alderuccio J. 06/14/2025; 4160985; PS1911
20. 2025 EHA. Huang H. 06/14/2025; 4161037; PS1963
21. 2025 EHA. Zeng Z. 06/13/2025; 4160375; PF969
22. 2025 EHA. LYU F. 06/13/2025; 4160319; PF913
23. 2025 EHA. Heß G. 06/14/2025; 4160946; PS1872
24. 2025 EHA. Cheah C. 06/13/2025; 4160289; PF883
25. 2025 EHA. Butaev L. 06/12/2025; 4162402; PB3329
26. 2025 EHA. Mizuta H. 06/13/2025; 4159415; PF1003
27. 2025 EHA. Sannikova M. 06/14/2025; 4160981; PS1907
28. 2025 EHA. Burke J. 06/14/2025; 4159309; S232
29. 2025 EHA. Buske C. 06/14/2025; 4160947; PS1873
30. 2025 EHA. Xu J. 06/14/2025; 4160976; PS1902
31. 2025 EHA. Wang W. 06/14/2025; 4160979; PS1905
32. 2025 EHA. Cheah C. 06/14/2025; 4160948; PS1874
33. 2025 EHA. Yang P. 06/12/2025; 4162256; PB3183
34. 2025 ICML, Juan-Manuel Sancho, 7.
35. 2025 ICML, Lorenzo Falchi, 27
36. 2025 ICML, L. Elizabeth Budde, 50.
37. 2025 ICML, Jennifer L. Crombie, 74.
38. 2025 ICML, Jeremy S. Abramson, 76.
39. 2025 ICML, L. Elizabeth Budde, 99.
(Source: “Oncology Outlook – Blood News” Editorial Department)
Disclaimer
All original articles signed belong to “Oncology Outlook”. Sharing and reprinting are welcome. This article is for medical and health professionals to understand the latest pharmaceutical information for reference only and does not represent the views of this platform. Such information cannot replace professional medical guidance in any way and should not be considered as diagnostic or treatment advice. If this information is used for purposes other than information, this site and the author bear no related responsibility.
