First Author: Zhongxiang Zhang
Corresponding Authors: Wang Erkang, Peng Zhangquan, Sun Baozhen, Ke Sun
Affiliations: Changchun Institute of Applied Chemistry, Chinese Academy of Sciences; Dalian Institute of Chemical Physics, Chinese Academy of Sciences; Jiangxi Normal University
Ethylene carbonate (EC) is one of the core solvents for lithium-ion battery electrolytes, playing a crucial role in lithium batteries due to its ionic conductivity and interfacial stability. However, its high melting point (36.4°C) limits the battery’s low-temperature performance. In contrast, propylene carbonate (PC) has garnered attention due to its lower melting point (-48.8°C). Unfortunately, the interface issues between PC and graphite anodes have hindered further applications of PC. Among various strategies to alleviate this limitation, selecting strongly solvating solvents to reduce the role of PC in the solvation shell has proven effective, yet the mechanism from the perspective of interfacial chemistry remains unexplored.
Here, Academician Wang Erkang from the Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Professor Peng Zhangquan from the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, and Ke Sun, Associate Professor Sun Baozhen from Jiangxi Normal University aimed to better understand the interaction mechanism between PC and graphite, particularly the reversible lithium intercalation into graphite in the presence of a large amount of PC. The authors selected NMP as a co-solvent with a high donor number (NMP, DN=27.3) paired with PC, which is commonly used as an industrial solvent in the preparation of lithium-ion battery electrodes but is rarely considered as a solvent in electrolytes.
In fact, based on the irreversible process of solvent co-intercalation in EC-free PC-based electrolytes, the authors confirmed the incompatibility of PC with graphite anodes. However, when NMP was used in conjunction with PC, an interesting synergistic effect was observed: the solvent co-intercalation disappeared, allowing Li+ to reversibly intercalate into graphite. Furthermore, by optimizing the volume ratio, the first cycle reversible capacity of graphite reached 331.2 mAh g-1, with an initial coulombic efficiency of 83.26%, approaching commercial standards, which is unprecedented performance for electrolytes based on PC and competing co-solvents. Additionally, solvation structure analysis indicated that NMP significantly reduced the content of PC in the Li+ solvation sheath, thereby avoiding destructive side reactions induced by PC, and further rationalized through theoretical calculations. More importantly, the authors confirmed through in situ Fourier-transform infrared spectroscopy (FTIR) and differential electrochemical mass spectrometry (DEMS) that NMP could reduce the reduction of PC at the graphite|electrolyte interface. Experimental results showed that the performance of Li-graphite half-cells based on PC/NMP coupled electrolytes was comparable to commercial EC electrolytes, and the low-temperature performance of LiNi0.8Co0.1Mn0.1O2-graphite full cells far exceeded that of commercial EC-based electrolytes, providing new opportunities for developing low-temperature electrolytes based on PC.
The related research results titled “Unlocking the Low-Temperature Potential of Propylene Carbonate to -30°C via N‑Methylpyrrolidone” were published in ACS Appl. Mater. Interfaces.
【Core Content】
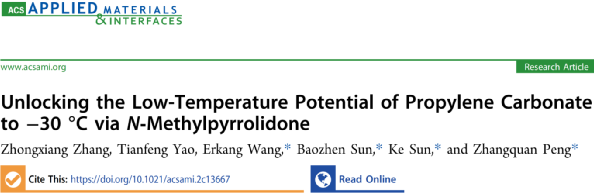
As shown in Figure 1a, the Li-graphite half-cell discharges in 1M LiPF6 PC at a voltage plateau of 0.9 V, indicating that it cannot form a stable SEI layer on graphite. Similarly, a similar situation is observed in the electrolyte with NMP as the solvent (Figure 1b); once the voltage reaches the threshold, its irreversible reduction of the solvent becomes inevitable. However, when the two unsuitable solvents are combined, a significant improvement in reversibility can be observed. In Figure 1e, in 1M LiPF6 PC/NMP=2:1 (v/v), the charge/discharge curves of the first three cycles of the Li-graphite half-cell closely match those in commercial EC-based electrolytes. After the first cycle, the reversible capacity of the battery was 331.2 mAh g-1, with an initial coulombic efficiency of 83.26%. These results clearly indicate that the “EC-like” electrochemical behavior has been reproduced in this combination of two individually unsuitable solvents.
To better visualize the differences in electrochemical performance, cyclic voltammetry (CV) curves confirm that the electrolytes with either PC or NMP as solvents exhibit irreversible reduction peaks, which gradually disappear in subsequent cycles, reflecting their completely irreversible nature. The difference is that in 1M LiPF6 NMP, the main irreversible cathodic peak voltage is 0.28 V, significantly lower than PC’s 0.52 V, indicating that NMP is more stable against electrochemical reduction than PC. Another difference is that before the main reduction process occurs below 0.5 V, two sub-peaks are distributed between 1.5−0.6V (peak voltages of 1.06 and 0.75 V), which may be related to the co-intercalation process of NMP, as evidenced by the partial reversibility of the Li-graphite battery in the 0.5-2.5V cycle. Additionally, some preliminary decomposition of NMP or anions PF6– may also be related, but these irreversible processes do not passivate the graphite anode.
Only in the PC-NMP system can three reversible cathodic/anodic peaks be observed for Li+ intercalation (de-intercalation) below 0.5 V (Figure 1f). An irreversible peak was observed at 0.76 V during the first cathodic scan, which may be related to the SEI formation process in this electrolyte system.
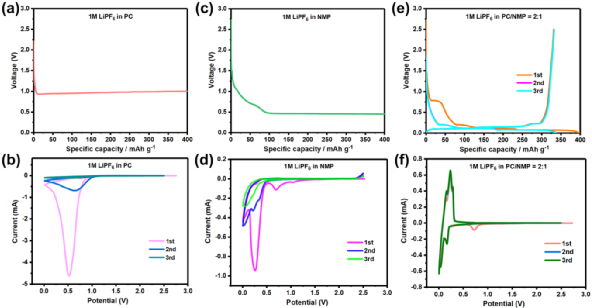
Figure 1. Discharge curves (C/20) and cyclic voltammetry (0.05 mV–1) curves of Li-graphite half-cells. (a, b) 1M LiPF6 PC, (c, d) 1M LiPF6 NMP, (e, f) 1M LiPF6 PC/NMP.
Simultaneously, scanning electron microscopy was used to observe the morphology of graphite anodes after cycling in three electrolytes. The original graphite particles were generally spherical with relatively smooth surfaces (Figure 2a-c). In 1M LiPF6 PC, after prolonged lithiation, the graphite flakes fell off, and the edges of the particles disintegrated, exposing the layered structure (Figure 2d-f). However, in 1M LiPF6 NMP, the surface morphology changes of lithiation in graphite were minimal, with no significant signs of flaking (Figure 2g-i), indicating that the reduction properties of NMP on the graphite surface differ from those of PC, likely producing only solid and liquid products. In the graphite anode cycled in 1M LiPF6 PC/NMP, as shown in Figure 2j−l, the surface of the lithiation remained intact, and the presence of NMP in the electrolyte effectively limited the gas evolution decomposition of PC, which is consistent with the good reversibility of the graphite anode in 1M LiPF6 PC/NMP.
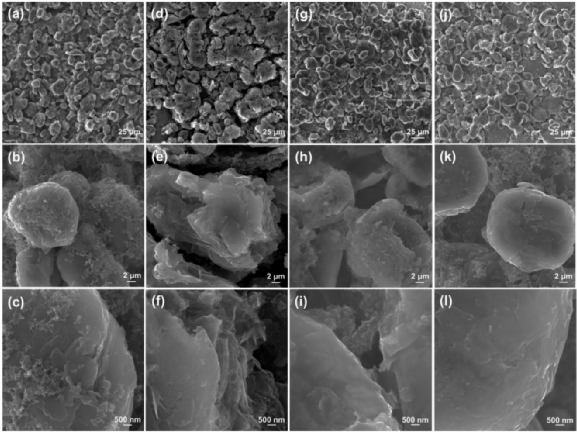
Figure 2. Morphology of graphite electrodes before and after low-rate cycling. (a-c) Original graphite electrodes, and after cycling in (d-f) 1M LiPF6 PC, (g-i) 1M LiPF6 NMP, (j-l) 1M LiPF6 PC/NMP.
In situ DEMS studies provided further insights into the solvent reduction mechanisms in the three electrolytes. In 1M LiPF6 PC, the only gas product detected was propylene, which began to evolve at 0.83 V and continued to rise until the end of the experiment, consistent with the mechanism of reduction decomposition of PC generating propylene. On the other hand, lithium propylene carbonate (LPDC) was the most important solid product in this reaction (Figure 2d-f). For 1M LiPF6 NMP, no gas was detected, which corresponds to the gas-free reduction of NMP suggested by SEM morphology studies. Notably, although propylene generation was still detectable in 1M LiPF6 PC/NMP, its onset voltage was delayed to 0.75 V, and its rapidly increasing trend suddenly reversed at 0.5 V, followed by a rapid decline. Clearly, after the addition of NMP, an effective mechanism has been established to passivate the graphite surface, minimizing the impact of gas evolution on the graphite surface. Importantly, post-discharge XPS of the graphite anode showed that the main reason for the failure of PC-based electrolytes was not due to LPDC and Li2CO3 being unable to construct a stable SEI, but rather the relatively insufficient products in excessive stripping and gasification reactions. In 1M LiPF6 PC/NMP, the reaction kinetics of different pathways were redistributed, increasing the content of LPDC, Li2CO3, and LiF, similar to the SEI formed in EC electrolytes, with NMP able to suppress the violent evolution/stripping of gases.
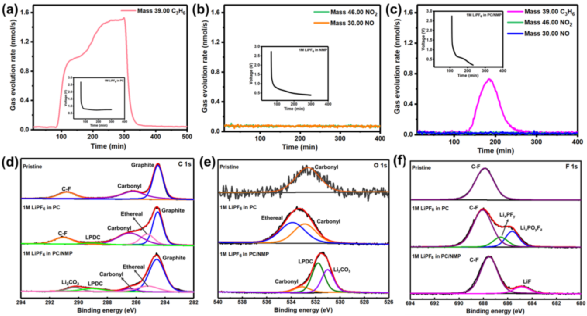
Figure 3. In situ DEMS of Li-graphite half-cells under different electrolytes.
Moreover, in 1M LiPF6 PC/NMP, the total number of coordinating solvent molecules did not change significantly compared to the two single solvent references. Since the solvent molecules coordinating Li+ have higher reduction enthalpy than free molecules, the reduction in the number of solvent molecules around Li+ corresponds to a decrease in the concentration of reactants for each solvent in the side reactions. Compared to 1M LiPF6 PC, the reduction of propylene is due to the decrease of PC molecules coordinated with Li+ in the PC/NMP sample. Additionally, existing studies indicate that as the number of PC molecules coordinated with Li+ decreases, the co-intercalation behavior of the solvent on the graphite surface is suppressed. Therefore, the reduction kinetics of both PC and NMP should be significantly reduced. The delayed reaction rate should be key to altering the reaction pathway of PC, favoring the formation of an ordered SEI.
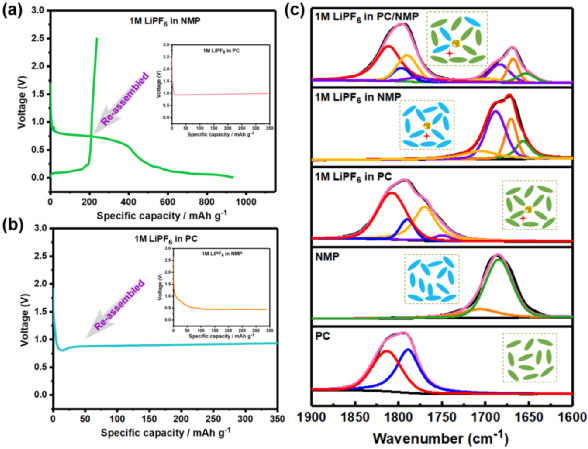
Figure 4. Evaluation of solvents at the interface and in the electrolyte. (a, b) Electrochemical behavior of graphite electrodes after surface solvent pre-treatment; (c) Structural analysis of solvents or electrolytes using FTIR.
To further rationalize the coordination chemistry revealed by infrared studies, DFT calculations were performed on the solvation energy and LUMO of the solvated complex Li+(PC)x(NMP)yPF6–. The results indicate that NMP molecules can replace PC in the solvation sheath, exhibiting stronger coordination ability than PC. Meanwhile, LUMO calculations show that PC is inherently more reductively active than NMP. However, only the coordinating solvent molecules are related to the reduction at the interface, as evidenced by the significant decrease in LUMO after Li+ coordination. Furthermore, LUMO has been identified to be primarily contributed by PC, consistent with the previous assumption that PC reduces to form SEI earlier than NMP before the unfavorable co-intercalation/reduction of NMP. In the PC/NMP electrolyte, the amount of PC in the solvation shell is nearly halved, which would neutralize or even offset the advantage of PC in the fundamental reaction kinetics.
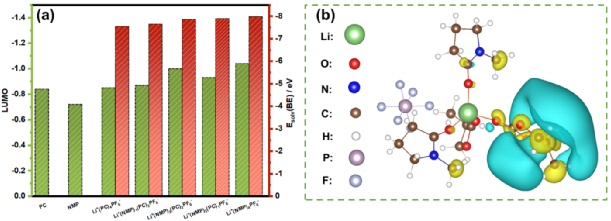
Figure 5. Simulation of the electrolyte.
To determine the reduction pathway of the PC-NMP binary solvent system in 1M LiPF6 PC/NMP, in situ FTIR studies were conducted on the graphite surface during the discharge process of this electrolyte. It was confirmed that in the PC/NMP electrolyte system, the controlled reduction of PC produced LPDC and Li2CO3 as necessary SEI components, which are also crucial for stabilizing the anode|electrolyte interface. In fact, it has also been reported that binary PC/DMSO systems can achieve a largely reversible reaction process, which may follow the same mechanism.
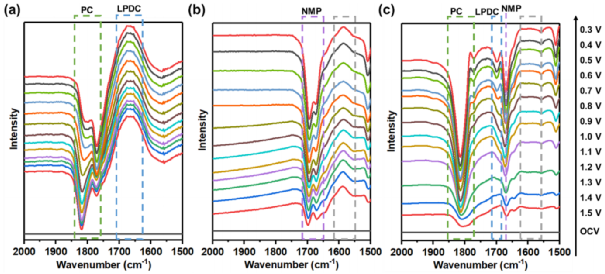
Figure 6. In situ FTIR spectra of different electrolytes.
In summary, this study investigated a novel electrolyte system based on a combination of PC and NMP, where the binary PC-NMP electrolyte system exhibits performance levels comparable to commercial EC-based electrolytes. Through a series of in situ and ex situ characterizations, the reduction mechanisms of PC and NMP individually in the new binary electrolyte system were revealed. Based on these studies, it was proposed that the presence of NMP in the electrolyte affects the reduction process of PC, i.e., NMP delays the gas evolution process and promotes the growth of LPDC and Li2CO3 on the graphite surface, which may constitute important components of the SEI layer, enabling graphite to withstand the detrimental co-intercalation of PC/NMP. Combining solvation structure analysis and theoretical calculations, it quantitatively reveals how NMP suppresses the decomposition kinetics of PC by altering the energetics of the reduction reactions, and demonstrates that PC can also serve as an important SEI-forming electrolyte solvent.
【References】
Zhongxiang Zhang, Tianfeng Yao, Erkang Wang,* Baozhen Sun,* Ke Sun,* Zhangquan Peng*, Unlocking the Low-Temperature Potential of Propylene Carbonate to -30°C via N‑Methylpyrrolidone,2022,ACS Appl. Mater. Interfaces.
https://doi.org/10.1021/acsami.2c13667
Lithium Metal Batteries: From Fundamental Research to Industrialization
2022-10-05

High-Reversible Lithium Host Materials for Addressing Volume Expansion Issues in Anode-Free Lithium Metal Batteries
2022-10-05

[Top Journal Review] Aqueous Multivalent Metal Ion Battery Layered Structured Compounds
2022-10-05
Nanjing University of Aeronautics and Astronautics Zhang Xiaogang, Bar-Ilan University Doron Aurbach AEM: Fe-Doped Induced Structural Transition of Manganese-Based Layered Oxides for Long Cycle Life High Stability Sodium Battery Cathodes
2022-10-05
Porous Carbon Anodes for Zinc-Ion Capacitors: Ion Space Limitation and Surface Chemical Properties
2022-10-05

Professor Zhang Shufang from Ludong University and Dr. Hu Yanqiang from Nantong University: High-Performance Perovskite Solar Cells Lead Leakage and Its Protection
2022-10-05

After thirty years of hard work, first published in Nature, then on the news broadcast! Molecular sieves finally “sieve” out a world in lithium batteries
2022-10-04

Zhang Jiguang et al. deeply analyze: The failure mechanism of micron silicon anodes and the impact of their pre-lithiation degree on battery performance
2022-10-04

Latest EES from Xi’an University of Technology: One stone three birds, sodium batteries with no anode, mechanical flexibility, and high energy/power density configuration
2022-10-04

Academician Cui Guanglei from the Chinese Academy of Sciences/Zhao Jingwen et al.: Heterogeneous Coordination Unlocks Zn2+ Conduction in Polymers
2022-10-04