Antibody-drug conjugates (ADC), also known as “magic bullets,” use monoclonal antibodies (Antibody) to deliver small molecule drugs (Drug) directly to tumor cells expressing specific antigens through a linker (Conjugate).
In recent years, ADC drugs have rewritten the treatment guidelines for blood cancers, breast cancer, ovarian cancer, and lung cancer, providing patients with significantly improved clinical data. The remarkable performance of ADCs has led to them being referred to as the next “tsunami” in cancer treatment.
How can we understand the changes brought about by ADC drugs? This article aims to provide a comprehensive overview of this type of medication.
Comprehensive Interpretation of ADC Drugs:
What Are They? What Are Their Advantages?
First, let me share a case:
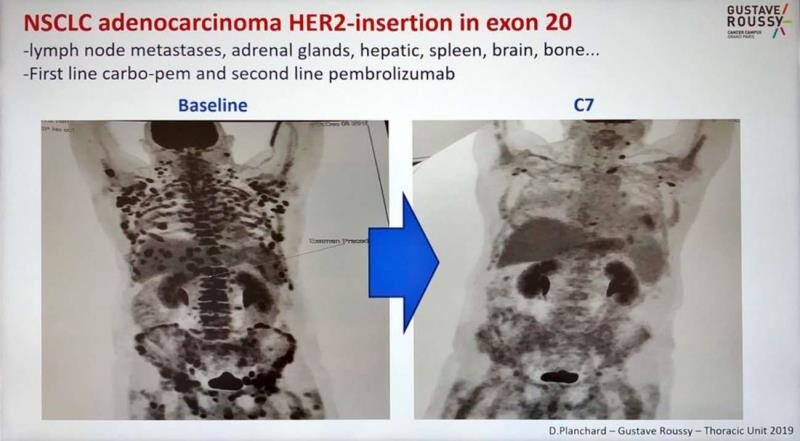
This is a comparison image of a lung cancer patient from Gustave Roussy Hospital in Paris, France, before and after treatment in 2019.
*Case source: https://twitter.com/dplanchard/status/1266701523809165313
The patient was diagnosed with non-small cell lung cancer and underwent chemotherapy (carboplatin + pemetrexed) and second-line immunotherapy (pembrolizumab), but neither treatment provided long-term benefits. As the disease progressed rapidly, the patient soon experienced metastasis to multiple organs, including lymph nodes, adrenal glands, liver, brain, and bones.
After a thorough analysis, the attending physician at Gustave Roussy Hospital discovered that the patient had a HER2 exon 20 insertion mutation, and recommended the patient to participate in a clinical trial of the ADC drug trastuzumab deruxtecan (T-DXd, DS-8201). In just two months, the patient’s condition significantly improved, exceeding the treatment expectations for previously treated lung cancer patients.
This illustrates the clinical potential of ADC drugs in cancer treatment. In recent years, with the continuous implementation of more clinical studies, ADC drugs have profoundly changed treatment outcomes for many patients. In clinical practice, ADCs are regarded as a new “fourth generation cancer therapy” following chemotherapy, targeted therapy, and immunotherapy.
In our current treatment options, killing cancer cells is not particularly difficult; the challenge lies in how to precisely kill cancer cells with minimal harm to normal tissues.
For example, chemotherapy is a highly effective method for eliminating cancer cells, but it inevitably damages normal cells, including bone marrow, gastrointestinal mucosa, germ cells, and neurons. Although we have corresponding measures to address the adverse effects of chemotherapy, it remains a significant burden for cancer patients. Furthermore, as we continue to study chemotherapy drugs, their efficacy has reached a bottleneck and cannot meet current clinical needs.
For cancer patients, achieving better survival benefits and improved quality of life from anti-cancer treatments is equally important. Better anti-cancer drugs are a common expectation among patients.
As early as 1913, Nobel laureate Paul Ehrlich proposed the concept of ADC drugs at the 17th International Medical Congress, referring to this idea as the “magic bullet.” The core of this concept is “precision” and “efficiency”: developing drugs that specifically kill cancer cells without affecting normal tissues.
After nearly a century of exploration, ADC drugs transitioned from concept to reality. In 2000, the first ADC drug, Mylotarg, was approved by the FDA for the treatment of acute myeloid leukemia.
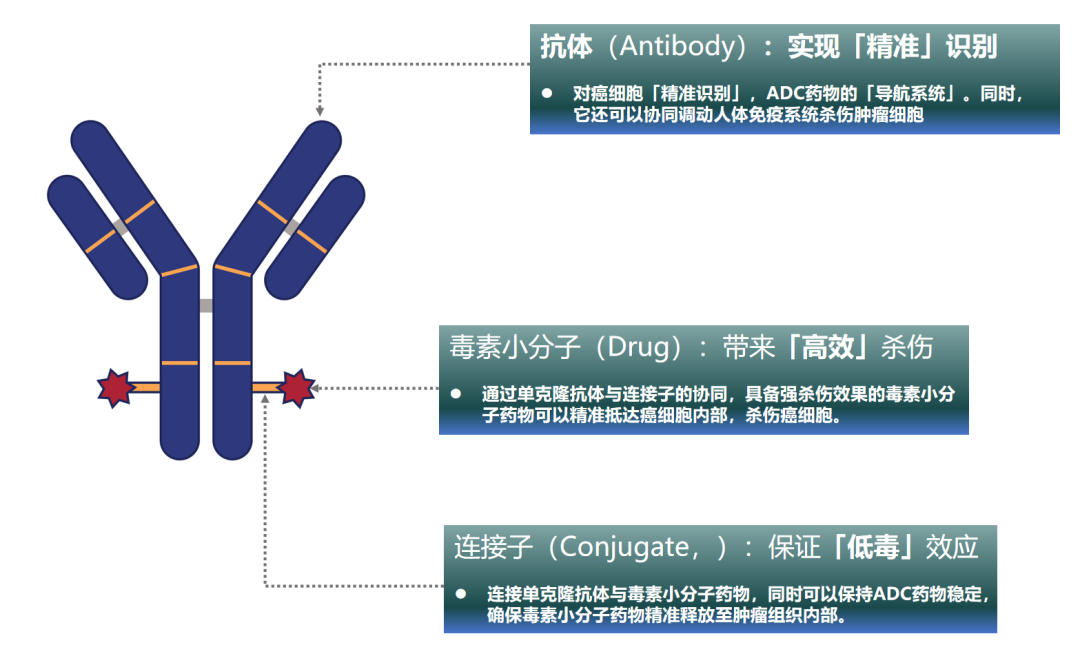
ADC drugs are formally called “Antibody Drug Conjugates” (ADC), and they primarily consist of three components:
Highly specific monoclonal antibodies (Antibody);
Highly stable linkers (Conjugate);
Highly effective small molecule drugs (Drug).
During treatment, the coordination of these three components allows ADC drugs to achieve a “precise, efficient, and low-toxicity” anti-cancer mechanism. Specifically:
The monoclonal antibodies are responsible for the ADC drug’s “precise recognition” of cancer cells, which can be understood as the navigation system of the ADC drug, guiding the toxic small molecules to kill cancer cells. Additionally, they can also stimulate the immune system to attack cancer cells;
The toxins are responsible for the “highly efficient killing” of cancer cells. Through the cooperation of monoclonal antibodies and linkers, powerful toxic small molecule drugs can accurately reach and kill cancer cells.
The linker binds the toxins and monoclonal antibodies together, serving as a bridge between them. The linker plays a crucial role; a good linker can maintain the stability of the ADC drug, ensuring that the toxins are not released during circulation in the bloodstream but only released within the cancer tissue, achieving the “low-toxicity” characteristic of ADC drugs.
With continuous technological breakthroughs, the third generation of ADC drugs has arrived, significantly enhancing their performance and further improving the efficacy and safety of clinical treatments. Since 2019, ADC drugs have rapidly developed, with 15 ADC drugs approved globally, seven of which have been approved in China.
ADC Drugs: The Next “Tsunami” in Cancer Treatment
HER2-positive non-small cell lung cancer patients
In August 2022, the FDA accelerated the approval of trastuzumab deruxtecan (T-DXd, DS-8201) (5.4mg/kg) for adults with unresectable or metastatic non-small cell lung cancer (NSCLC) with HER2 mutations who had previously received systemic treatment. Trastuzumab deruxtecan became the first and currently the only approved ADC drug in the field of lung cancer.
In the global multicenter clinical study of trastuzumab deruxtecan (DESTINY-Lung02), the 5.4mg/kg dose demonstrated excellent clinical efficacy in treated HER2-mutated non-small cell lung cancer patients, bringing very encouraging news to patients. After using T-DXd, the objective response rate (ORR) reached 49%, with most patients experiencing tumor shrinkage, while the median progression-free survival (mPFS) was 10 months, and the median overall survival (mOS) reached 19.5 months, extending the survival time of patients.
Moreover, a domestic bridging study conducted for Chinese patients (DESTINY-Lung05) also reported similar encouraging results, with ORR reaching 58.3%. This indicates that trastuzumab deruxtecan can provide significant treatment effects for patients both internationally and in China.
Currently, the 2023 version of the National Comprehensive Cancer Network (NCCN) guidelines recommends trastuzumab deruxtecan as the preferred treatment for advanced or metastatic HER2-positive patients whose disease progresses during or after initial systemic therapy.
In China, the Chinese Society of Clinical Oncology (CSCO) guidelines for non-small cell lung cancer have also included trastuzumab deruxtecan as a recommended option for post-line treatment of stage IV HER2-mutated NSCLC (Level II recommendation). The excellent clinical performance of trastuzumab deruxtecan for HER2-mutated lung cancer patients significantly addresses their clinical needs.
More Comprehensive and Effective: TROP2 ADC Drugs Show Promise in Advanced Lung Cancer Treatment
In addition to trastuzumab deruxtecan, more ADC drugs targeting lung cancer are making breakthroughs. Among them, Dato-DXd has become the first and currently the only targeted TROP2 ADC drug to achieve positive results in the primary endpoint of Phase III clinical trials, promising more effective treatment options for patients in the near future.
In the global multicenter Phase III randomized controlled study TROPION-Lung01, Dato-DXd demonstrated significantly better efficacy and safety compared to standard chemotherapy in treated patients with advanced non-squamous non-small cell lung cancer:
The median progression-free survival improved by nearly two months, at 5.5 months vs. 3.6 months, with overall survival also showing clear clinical benefits. In terms of objective response rate, 31% of patients in the Dato-DXd group experienced significant improvement, compared to only 13% in the standard treatment group, nearly 2.5 times higher; additionally, four patients receiving Dato-DXd achieved complete remission, meaning their target lesions completely disappeared.
In terms of safety, Dato-DXd also showed advantages, with a reduced incidence of ≥ grade 3 drug-related adverse events compared to the standard treatment group (25% vs. 41%), indicating that Dato-DXd is not only more effective but also safer, capable of extending patients’ survival and improving their quality of life.
Looking Ahead: More ADC Drugs Bring Hope
According to a report released by Morgan Stanley last year, there are currently at least 1,400 ADC clinical trials ongoing. Several “star ADC drugs” targeting HER3, c-MET, B7-H3, and other targets have made preliminary breakthroughs and are expected to benefit a wide range of lung cancer patients soon.
As ADC drugs are currently “riding the wave,” clinical research on ADC drugs for advanced lung cancer is rapidly developing. Furthermore, in the future, ADC drugs may also be used for neoadjuvant and adjuvant therapies in early-stage cancer patients, helping patients achieve clinical cures; additionally, combinations with current standard treatments, such as “ADC + immunotherapy, ADC + targeted therapy, ADC + ADC,” may also play significant roles in cancer treatment. Moreover, with advancements in drug technology, more iterations of ADC drugs are expected to further enhance clinical efficacy and safety, ushering in a new era of ADC therapy.
We look forward to that day.
For more information on ADC drugs, please follow the ADC information zone.