Xie Y, Rufo J, Zhong R, Rich J, Li P, Leong KW, Huang TJ. Microfluidic Isolation and Enrichment of Nanoparticles. ACS Nano. 2020 Dec 22;14(12):16220-16240. doi: 10.1021/acsnano.0c06336. Epub 2020 Nov 30. PMID: 33252215; PMCID: PMC8164652.
In recent decades, the application of nanoparticles has increased in various applications, ranging from efficient electronics to targeted drug delivery. Recently, microfluidic technology has become an important tool for the separation and enrichment of nanoparticle populations with uniform characteristics (such as size, shape, and charge) due to its precision, versatility, and scalability. However, due to the numerous microfluidic techniques available, determining the most suitable method for separating or enriching target nanoparticles can be challenging. In this review article, we investigate microfluidic methods for nanoparticle separation and enrichment based on their potential mechanisms (including acoustic flow, dielectrophoresis, filtration, deterministic lateral displacement, inertial microfluidics, optical flow, electrophoresis, and affinity-based methods).
For decades, nanoparticles have attracted significant attention from the scientific community due to their unique chemical and physical properties. Nanoparticles include naturally occurring “soft nanoparticles” such as proteins and DNA, as well as synthetic “hard nanoparticles” such as gold and silver. Nanoparticles are widely used in fields such as catalysis, electronics, biology, and medicine, with a growing market size and an increasing number of scientific publications. The uniform size, shape, charge, and chirality of nanoparticles are crucial to their performance, especially in biosensing and therapeutic applications. However, impurities can easily arise during manufacturing, necessitating the development of post-manufacturing methods to enhance uniformity. Separation and enrichment are key steps in improving uniformity. Microfluidic technology, as an emerging technology, has successfully separated and enriched various nanoparticles.
Separation and enrichment are two major steps in enhancing the uniformity of nanoparticles. Separation acts as a selective barrier process that allows one component to pass freely while retaining or redirecting other components. After separation, the concentration of nanoparticles may decrease due to particle loss or the introduction of additional fluid, thus requiring a concentration procedure to enhance the concentration of specific nanoparticles. Enrichment can also increase the local concentration of nanoparticles for easier detection. Notably, techniques such as density gradient centrifugation can achieve both separation and enrichment simultaneously.
Microfluidic technology is an emerging technology for the separation and enrichment of nanoparticles, encompassing multiple disciplines including physics, chemistry, engineering, and nanotechnology. This paper reviews the latest advancements in microfluidic nanoparticle separation and enrichment, focusing on the technical mechanisms of acoustics, optics, dielectrophoresis, and filtration, and discusses solutions for specific particle characteristics as well as the advantages and disadvantages of each technique. The article focuses on microfluidic devices with characteristic lengths of approximately 100 μm or smaller, excluding other micron-scale techniques such as high-performance liquid chromatography and capillary electrophoresis.
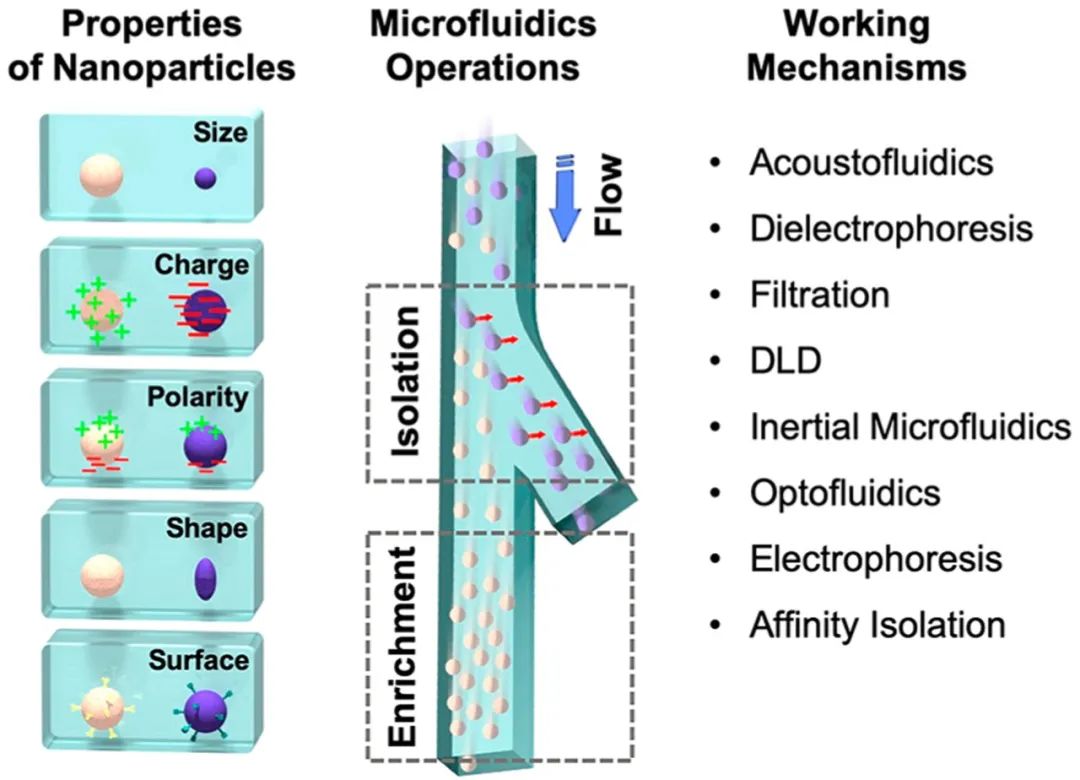
Figure 1.
Illustration showing the processes and mechanisms of nanoparticle separation and enrichment in microfluidic systems.
Acoustic Flow
The acoustic flow method combines acoustic manipulation with microfluidic devices to effectively separate nanoparticles. In an acoustic field, particles are deflected based on material properties and size. Studies have found that the acoustic field can capture, focus, and pattern nanoparticles. Wu et al. demonstrated the separation of polystyrene particle mixtures using acoustic flow at flow rates of 4 μL/min and 12 μL/min, achieving a recovery rate of 90.7% for 110 nm particles.
As the deflection distance of the particles increases, researchers successfully separated polystyrene particles sized 300 nm and 500 nm. The power and frequency ranges used in the acoustic flow method are similar to those in ultrasound imaging, exhibiting good biocompatibility. Based on this, Wu et al. further developed an acoustic flow exosome separation technique that integrates two modules: the removal of larger blood components and the separation of extracellular vesicles, achieving exosome separation with a purity of 98.4% and a blood cell removal rate exceeding 99.999%. Other extracellular vesicles can also be separated using acoustic flow. Lee et al. proposed an “acoustic nanofilter” system that continuously separates microvesicles with size specificity. This filter separates nanometer-sized (<200 nm) vesicles from cell culture media and red blood cell products, achieving separation rates of over 90% (Figure 2C, D).
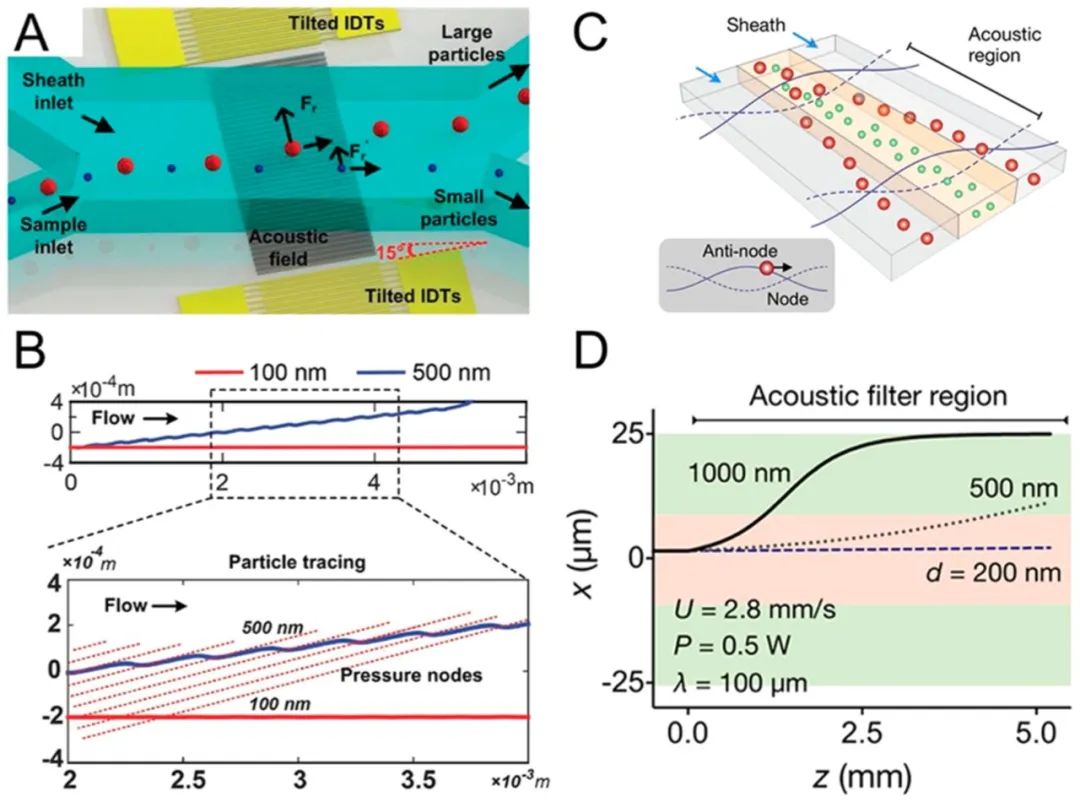
Figure 2.
Acoustic nanoparticle separation. (A) Schematic of nanoparticle separation and (B) simulation, with the standing surface acoustic wave field tilted relative to the microfluidic channel. (C) Schematic of separating nanoparticles using a standing surface acoustic wave field parallel to the microfluidic channel and (D) simulation. Reproduced from ref77. Copyright 2015 American Chemical Society.
The acoustic flow method can enrich, concentrate, or capture nanoparticles through acoustic radiation forces, acoustic flows, or both. Mao et al. designed an acoustic flow chip to concentrate nanoparticles at the center of a glass capillary. Collins et al. introduced highly focused surface acoustic waves at frequencies ranging from 193 to 636 MHz, generating localized acoustic vortex flows at the microfluidic length scale. High-frequency surface acoustic waves generate localized vortices in microfluidics to capture nanoparticles. When the acoustic frequency reaches gigahertz, Cui et al. demonstrated a method to concentrate 87 nm particles through acoustic vortex flow. Furthermore, Reyes et al. focused nanoparticles using radiation forces in acoustic standing waves, successfully achieving agglomeration of 200 nm gold nanoparticles.
The acoustic flow method separates biological nanoparticles without the need for high shear, high temperature, or special media. This method allows for label-free separation based on size or other physical property differences. Microfluidic channels facilitate precise fluid control in acoustic operations. In microfluidic devices, low Reynolds numbers (laminar flow) achieve high separation resolution, superior to bulk fluids. Acoustic flow nanoparticle separation is based on particle size differences, potentially enabling integrated in-system isolation and enrichment. Existing acoustic flow devices are limited by bulky, expensive, and specialized electronic equipment, restricting their industrial applications. In recent years, research has focused on developing low-cost, open-source alternatives for acoustic flow devices.
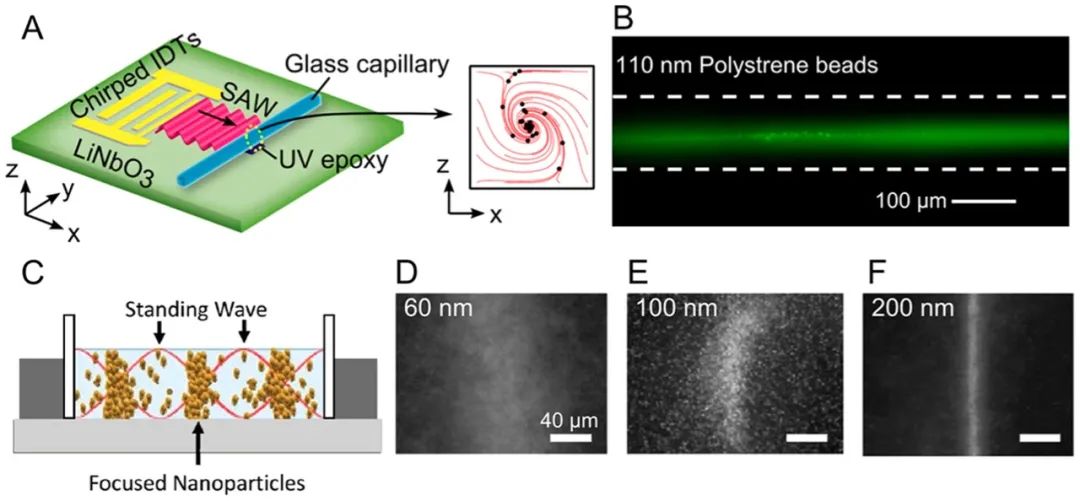
Figure 3. Acoustic enrichment of nanoparticles. (A) Schematic of concentrating 110 nm polystyrene beads in a capillary and (B) experimental demonstration. (C) Schematic showing how to concentrate nanoparticles at pressure nodes using primary acoustic radiation forces. Experimental images show how concentration effects correlate with size.
Microfluidic Dielectrophoresis
Microfluidic dielectrophoresis (DEP) describes the movement of dielectric particles caused by polarization effects in non-uniform electric fields within microfluidic devices.85–90DEP has been widely studied for the separation and enrichment of various nanoparticles.87,91-97For example, Viefhues et al.98developed a DEP-based device for separating polystyrene nanoparticles of 20 and 100 nm. They found that 85-100% of the larger nanoparticles were deflected, and it was anticipated that nanoparticles with a diameter difference of approximately 30% could be effectively separated. Zhao et al.99used non-uniform direct current electric fields (DC-DEP) for DEP separation of nanoparticles. In this method, the conductivity of the suspension solution was adjusted so that polystyrene nanoparticles of a given size experienced positive DEP, while another size experienced negative DEP. Using this method, separation of 51 and 140 nm nanoparticles as well as separation of 140 nm and 500 nm nanoparticles was demonstrated (Figure 4A–C). Sonnenberg et al. used a similar mechanism with a working frequency of 20 V peak-to-peak and 10 kHz to separate DNA from blood cells, where high molecular weight DNA and nanoparticles were concentrated into high field regions through positive DEP, while blood cells were concentrated into low field regions through negative DEP. Using this method, high molecular weight DNA was detected at 260 ng/mL, which is an appropriate range for DNA biomarkers (Figure 4D–F). Krishnan et al.101demonstrated the separation of 10 nm polystyrene nanoparticles, 60 nm DNA-derivatized nanoparticles, and 200 nm nanoparticles. They showed the feasibility of this technique, even in high conductivity physiological solutions.
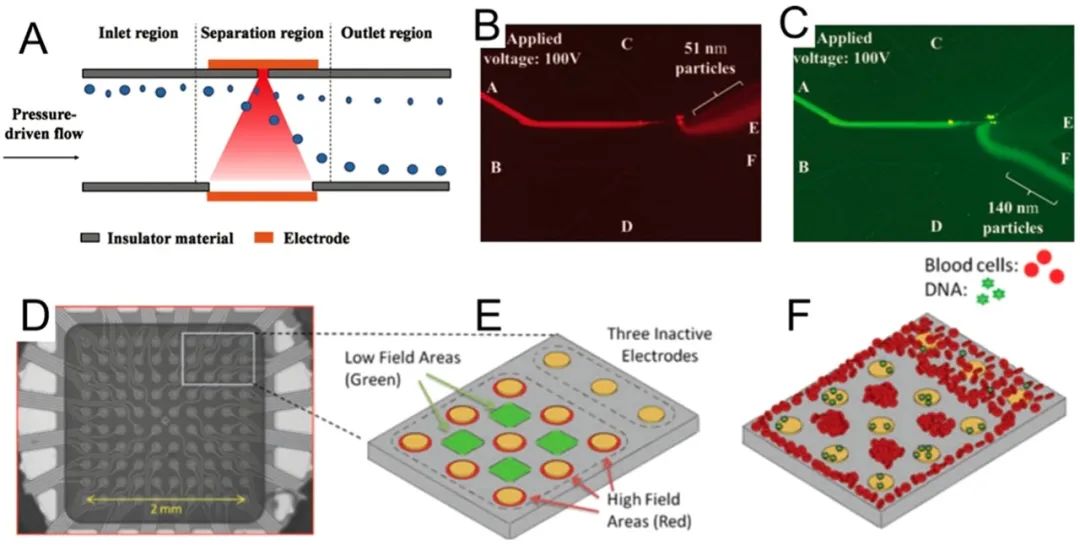
Figure 4.
Dielectrophoretic nanoparticle separation. (A) A dielectrophoretic method based on nanopore openings for separating nanoparticles sized (B) 50 and (C) 140 nm. Reproduced with permission from ref 99. Copyright 2016 Royal Society of Chemistry. (D) Microscopic image and (E) schematic of a dielectrophoretic microarray device that operates in a semi-continuous manner for (F) separating DNA and nanoparticles from blood. Reproduced with permission from ref 100. Copyright 2012 Wiley.
In addition to separation, nanoparticles can also be enriched through DEP capture mechanisms.102-106Cheng et al.107developed an electrode array to generate DEP forces and aggregate bacteria with silver nanoparticles. They used this method to rapidly identify bacteria in diluted blood through surface-enhanced Raman spectroscopy (SERS) (Figure 5A,B).B). Han et al.108superimposed AC DEP and electroosmosis between two coplanar electrodes to concentrate bacteria, viruses, and proteins. They enriched nanoparticles sized MS2 viruses and troponin I antibodies. Yeo et al.109eliminated the limitations of microfluidic devices and developed a dendritic, multi-tipped nanoscale probe (i.e., dendritic nanoscale probe) for DEP-based viral particle concentration. They demonstrated that dendritic nanoscale probes could detect as low as 10 T7 phage particles per mL (20 particles in a 2 μL sample volume) within 5 minutes (Figure 5C,D).D
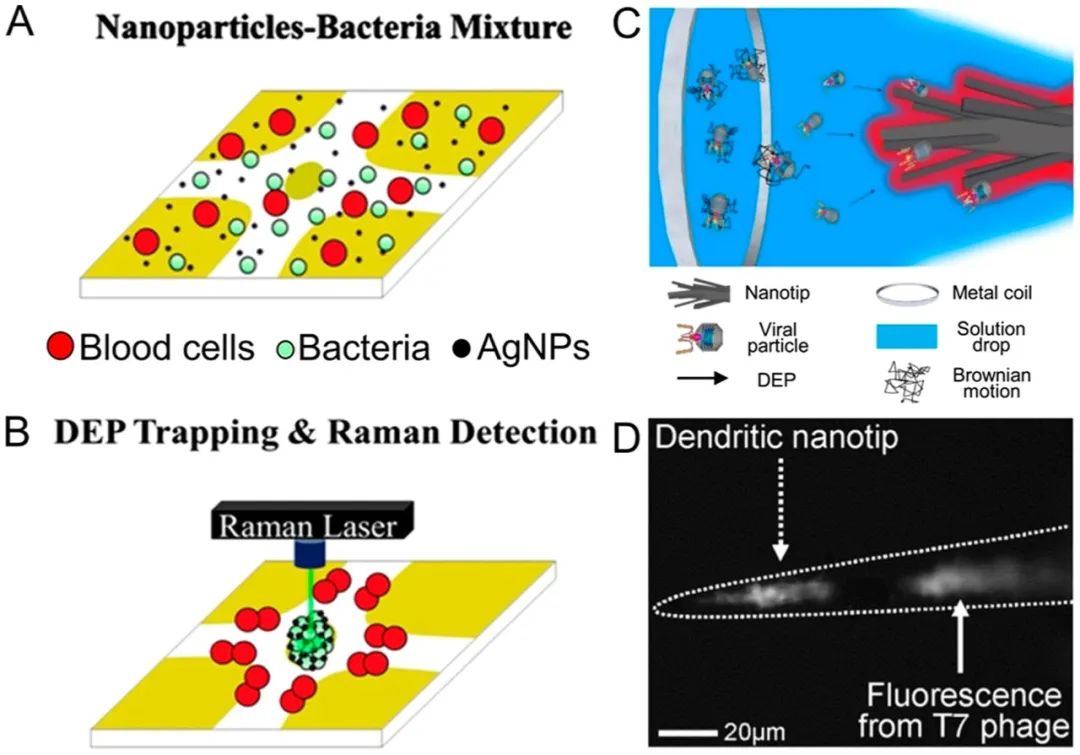
Figure 5.
DEP-supported nanoparticle enrichment. (A, B) Schematic of the DEP concentration of bacteria and Ag nanoparticles, with electrodes deposited on a glass substrate. Reproduced with permission from ref 107. Copyright 2014 Springer. (C) Schematic and (D) microscopic image of virus (i.e., T7 phage) DEP concentration on dendritic nanoscale probes. Reproduced with permission from ref 109. Copyright 2013 IOP Publishing.
Microfluidic DEP has multiple advantages.88,90,110,111First, DEP forces do not require target particles to carry a charge; they can work with both charged and neutral nanoparticles. Second, DEP forces require non-uniform electric fields, which can be easily achieved through modern microfluidic design and electrode fabrication techniques. Additionally, the laminar flow characteristics in microfluidic channels provide excellent flow control compared to bulk devices. However, microfluidic DEP has several limitations that still need to be addressed. First, although DEP-based methods have been widely applied, their underlying mechanisms are not yet fully understood. The dielectric properties of materials, the angular frequency of the applied electric field, and the charge and size of the particles all influence the separation outcomes.90Therefore, predicting the performance of DEP separation before conducting separation experiments is not straightforward. Second, many DEP-based biological applications require specific media with predefined conductivities, which may affect the biological functions of the targets. Furthermore, applying external voltages to drive particle movement may induce electrothermal flow and Joule heating,112which can disrupt the separation process and integrity of biological nanoparticles.
Microfluidic Filtration
Filtration is one of the most widely used industrial nanoparticle separation methods in water treatment, fractionating nanoparticles through direct physical barriers. Its microfluidic counterpart113-117is based on similar fundamental concepts but also aims to handle small volumes of samples. Traditionally, microfluidic filtration is at least partially categorized into field-flow fractionation,118which is defined by the characteristics of the flow field rather than the nature of the filtration. In this regard, asymmetric flow field flow fractionation (AF4) products can be used to separate various proteins, liposomes, emulsions, viruses, polysaccharides, metal, and polymer nanoparticles.119-122Recently, Zhang et al.123identified two exosome subpopulations (large exosome vesicles of 90-120 nm and small exosome vesicles of 60-80 nm) using AF4, and discovered a rich group of non-membranous nanoparticles called “exosomes” (~35 nm). In addition to commercial systems, emerging concepts of microfluidic filtration are also rapidly developing. For example, Davies et al.124developed a microfluidic filtration system with a porous polymer monolithic membrane in a polymethyl methacrylate microfluidic chip through UV polymerization to separate vesicles from whole blood samples. Filtration is driven by pump injection or DC electrophoresis. Liang et al.125developed a dual-filtration microfluidic device for separating and enriching extracellular vesicles ranging from 30-200 nm in size from urine. They demonstrated a separation rate of 80% for extracellular vesicles from T24 cell culture and urine samples (Figure 6A,B).B), and applied their method for bladder cancer detection. We do not discuss nanoparticle enrichment based on filtration, as separation through filtration is typically also an enrichment process.
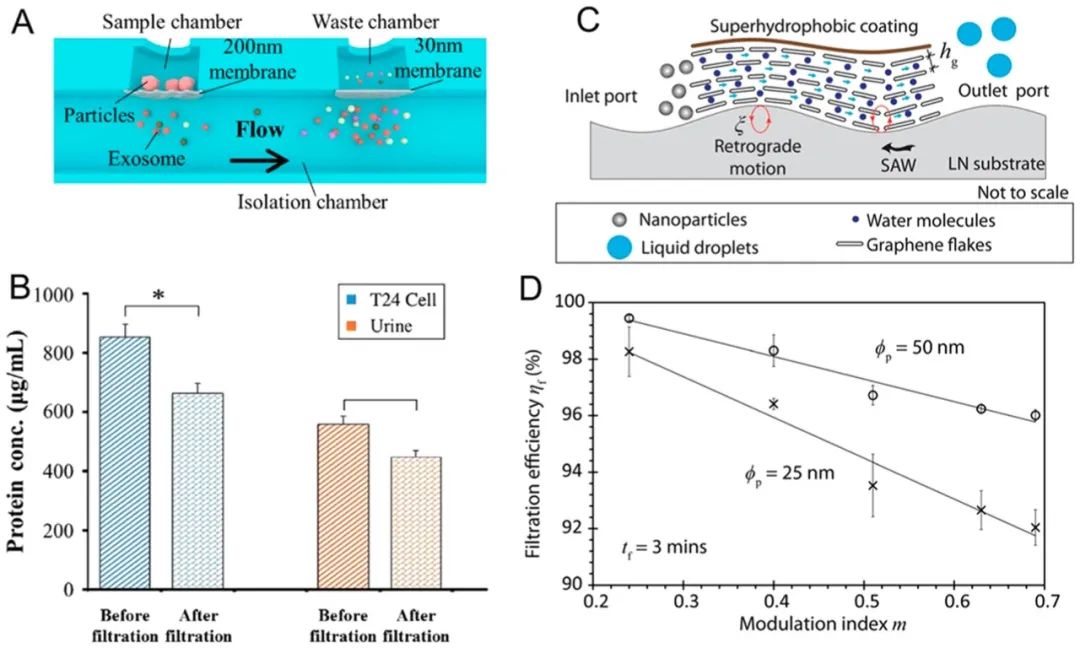
Figure 6.
Filtration-based nanoparticle separation. (A, B) Dual-filter microfluidic device for separating extracellular vesicles from urine and cell culture. Reproduced with permission from ref 126. Copyright 2017 Royal Society of Chemistry. (C, D) Integrating surface acoustic waves with graphene filters to isolate nanoparticles suspended in water. Reproduced with permission from ref 125. Copyright 2017 Nature Publishing Group.
As a direct derivative of filtration, microfluidic filtration has some of the same inherent limitations, and researchers are working to overcome these limitations. For instance, nanoparticles passing through nanoporous filters often require high energy and may clog or block the membrane. To address these two issues, Ang et al.126used surface acoustic waves (SAW) to enhance the transmission of graphene films. They achieved 100% filtration efficiency for micrometer-sized particles and 95% filtration efficiency for particles as small as several tens of nanometers, demonstrating the ability to separate nanoparticles with diameters of 25 and 50 nanometers. To avoid membrane clogging, backwashing can be performed simply by reversing the flow induced by SAW to flush out the embedded nanoparticles. After SAW-induced backwashing, filtration efficiency reached 98% (Figure 6C,D).D). Another limitation of filtration is that once a membrane is prepared, it is challenging to change the pore size. To overcome this limitation, Haefner et al.127demonstrated a method to adjust the size exclusion function of nano/micro filters based on poly(N-isopropylacrylamide) (PNIPAAm) in 2D and 3D microfluidic systems. The pore size can be adjusted from nano to micro by responding to the contraction or expansion of organic solvents.
Microfluidic Deterministic Lateral Displacement (DLD)
Microfluidic DLD utilizes the arrangement of pillars to control the trajectories of particles, facilitating the separation of particles larger and smaller than a critical diameter.128,129DLD provides excellent size resolution for nanoparticle separation. For example, Huang et al.130separated microspheres of 0.8, 0.9, and 1.0 μm within 40 seconds, achieving a resolution of ~10 nm at a flow rate of approximately 100 μm/s. They also successfully separated particles ranging from 1000 to 600 nm (Figure 7A,B).B). Later, Santana et al.131used DLD to separate extracellular vesicles derived from cancer cells (BxPC-3 cells); they achieved a yield of 39% with a purity of 98.5%. Although the yield was not satisfactory, the high purity could benefit downstream cancer diagnostic applications. Since its inception, researchers have been dedicated to improving the performance of DLD in nanoparticle separation. First, researchers created “nano DLD” to separate smaller nanoparticles. Wunsch et al.132used manufacturable silicon to produce nano-scale DLDs, with gap sizes ranging from 25 to 235 nm. They demonstrated size-based separation of nanoparticles between 20 and 110 nm; they also separated exosomes based on size (average 60-80 nm, ranging from 20 to 140 nm), which are necessary precursors for single-particle exosome analysis (Figure 7C,D).D). Additionally, researchers enhanced the dynamic range of nanoparticle separation in DLD by actively manipulating particle size. For example, by varying the ionic concentration of various buffer solutions, Zeming et al.133were able to adjust the effective size of nanoparticles. This, in turn, altered the magnitude of the electrostatic forces between the nanoparticles and the walls of the DLD device (Figure 7E). They demonstrated dynamic control of the separation spectrum of particles ranging from 51 to 1500 nm in continuous flow; this separation spectrum is approximately 12 times larger than the spectrum of traditional DLD separations.
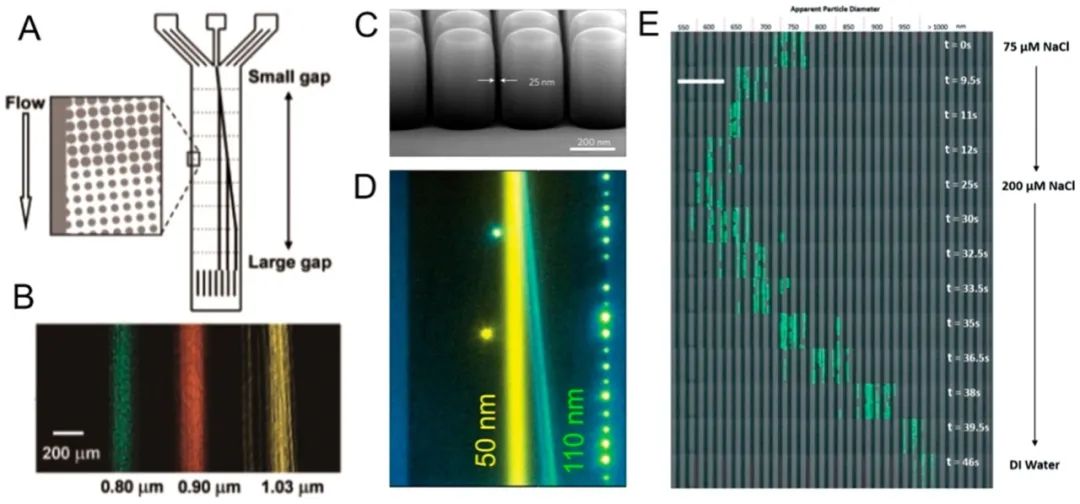
Figure 7.
DLD-supported nanoparticle separation. (A, B) DLD separation of nanoparticles of three sizes. Reproduced with permission from ref 130. Copyright 2004 American Association for the Advancement of Science. (C, D) Nano-DLD can separate nanoparticles with size differences of less than 100 nm. Reproduced with permission from ref 132. Copyright 2016 Nature Publishing Group. (E) Adjusting the size of nanoparticles in DLD separation by adding solvents. Reproduced with permission from ref 133. Copyright 2016 Royal Society of Chemistry.
While DLD is primarily used for separation, it is also utilized for nanoparticle enrichment. It is based on the mechanism of nanoparticle focusing within pillar arrays. For example, Chen et al.134reported an increase in the concentration of DNA (diameter ~250 nm) by 87 times at a flow rate of 0.25 μL/h (flow rate of 40 μm/s). Specifically, they used polyethylene glycol (PEG, 10% w/v) to increase the shear modulus and compact the DNA molecules to enhance separation performance. They claimed that DNA purified from enzymatic reactions could be integrated to produce next-generation DNA sequencing libraries.
DLD methods exhibit good separation performance, high resolution, and the elimination of external forces, making them suitable for both nanoparticle separation and enrichment. Integrating pillar structures into microfluidic devices allows for precise control over the interactions between particles, flow, and microstructural features. However, current DLD methods still have some inherent limitations: first, the fluid volumes processed by DLD are typically very small (1-10 μL/min). Second, the devices can easily become clogged by larger particles and impurities. To circumvent the first limitation, DLD nanoparticle separation is mainly used for diagnostic purposes involving extracellular vesicles and DNA, where the high concentration of biomarkers means that throughput is not a major concern. To address the second issue, DLD devices can be integrated with other mechanisms to preprocess samples and remove larger objects that may clog the devices.
Inertial Microfluidics
Inertial microfluidics135–140nanoparticle separation methods utilize the inertial migration of particles in microfluidic channels, which have pre-designed shapes (e.g., straight channels, helical channels, or wavy channels) and cross-sectional geometries (e.g., rectangular, circular, or triangular) to focus nanoparticles at different locations. The effects of inertial focusing are driven by shear gradient lift and wall effect lift, and depend on the design of the channel, flow rate, and particle size.141Thus, inertial focusing can separate nanoparticles of various sizes. In this regard, Bhagat et al.142demonstrated the extraction of 590 nm polystyrene particles from a mixture of 1.9 μm and 590 nm particles in a straight microfluidic channel with a rectangular cross-section (Figure 8A,B).B.
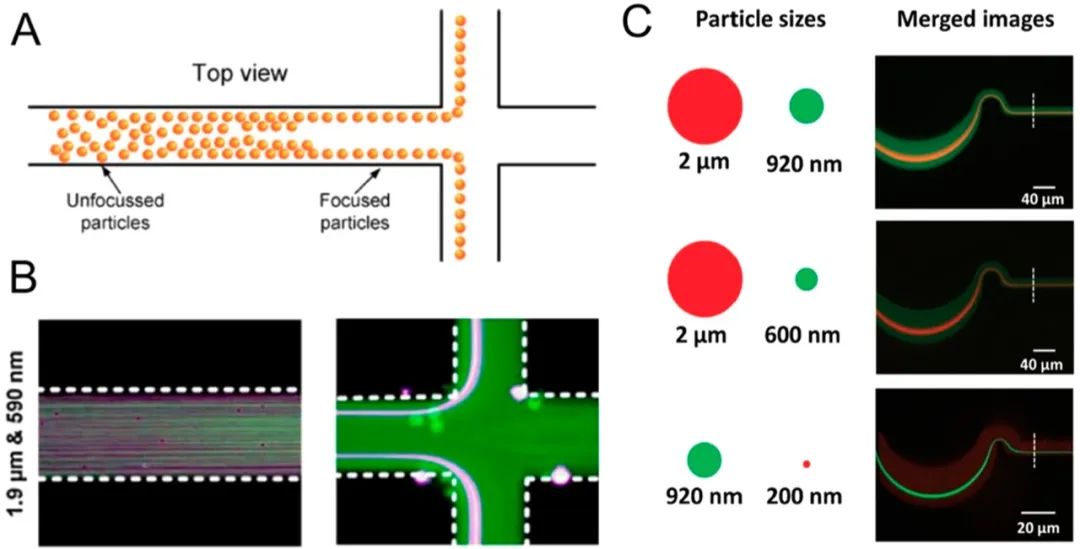
Figure 8.
Inertial microfluidic nanoparticle separation. (A, B) Nanoparticle separation in a straight rectangular microchannel based on shear-induced inertial lift. Reproduced with permission from ref 142. Copyright 2009 Springer. (C) High-throughput nanoparticle separation in a helical channel. Reproduced with permission from ref 145. Copyright 2017 Wiley.
To better control particle trajectories in microfluidic channels, polymers have been introduced as suspensions carrying nanoparticles. The particles laterally migrate in the flow direction driven by the elastic forces generated by the deformation of polymer chains, achieving “viscoelastic focusing.”141Using viscoelastic focusing in nanoparticle separation, Liu et al.143separated 100 and 2000 nm polystyrene particles and λ-DNA/platelet binary mixtures in a helical microfluidic device in polyethylene oxide solution, achieving >95% separation efficiency. Liu et al.144designed a high aspect ratio channel measuring 50 μm high and 20 μm wide; they separated exosomes from cell culture media with high purity (>90%) and yield (>80%). They also demonstrated the separation of 100 nm and 500 nm polystyrene nanoparticles. They could adjust the cutoff size of nanoparticles by varying the concentration of polyethylene oxide in the suspension medium. Wang et al.145further optimized the design of wavy channels, using thermosetting polyester instead of polydimethylsiloxane (PDMS). By doing so, they reduced channel cross-sectional deformation caused by pressure and maintained focusing effects at higher flow rates of up to 1400 μL/min. They demonstrated separation between 920 nm and 200 nm polystyrene microspheres, although the separation performance under these conditions needs further characterization (Figure 8C.).
Nanoparticles can also be enriched in microchannels through inertial focusing and viscoelastic focusing.138,140Kim et al.146designed a straight channel with a rectangular cross-section, which they used to focus fluorescent submicron polystyrene beads of diameters 500 and 200 nm along the centerline of the microchannel, adding 500 ppm poly(ethylene oxide). They also focused flexible DNA molecules (λ-DNA and T4-DNA), which have rotational radii (Rg) of approximately 0.69 and 1.5 nm, respectively. Zhou et al.147integrated the focusing and separation processes of exosomes into a single device (Figure 9). They periodically reversed the Dean flow generated by the repetitive wavy channel structure, causing larger particles (e.g., large extracellular vesicles) to concentrate at the centerline, while smaller particles (e.g., exosomes) were focused along the edges for separation. After a single separation process, their exosome purity reached 92.8%.
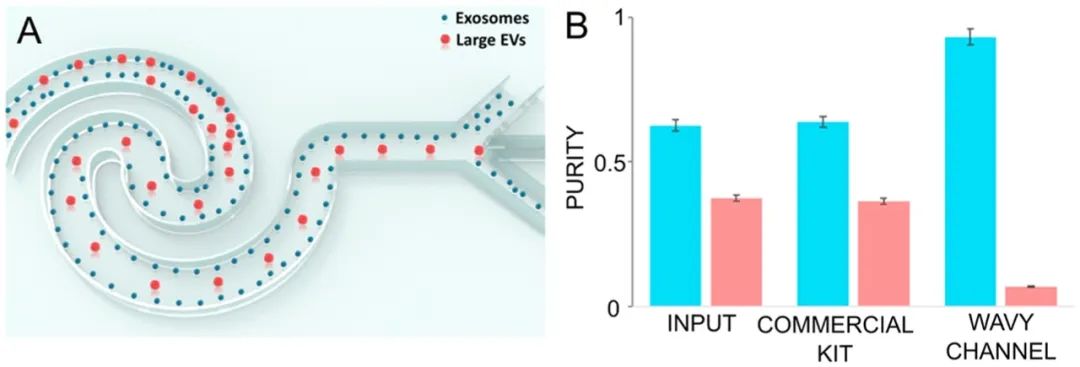
Figure 9.
Exosome enrichment and separation based on inertial microfluidics. Reproduced from ref147. Copyright 2019 American Chemical Society.
Compared to methods that utilize external forces such as optics, acoustics, or electronics, inertial microfluidic nanoparticle separation and enrichment techniques have advantages in ease of use, no need for external drives, and stable performance after optimizing operational parameters. In contrast to DLD methods, removing pillars alleviates concerns about channel clogging and improves throughput. Microfluidic channels also allow for precise fluid control over the number of capillaries, Reynolds number, and Peclet number,135which is challenging in bulk devices. Nonetheless, inertial microfluidic operations typically work at high flow rates, where shear forces may pose potential damage to biological nanoparticles. While there are no detailed studies reporting this effect, further validation may be needed to confirm the biocompatibility of inertial techniques.
Optofluidics
Integrating optics with microfluidics (i.e., optofluidics)148-153provides a powerful tool for high-resolution separation and enrichment of nanoparticles.154-161Nan et al.162produced isolated metal nanoparticles with dynamic and tunable optical forces generated by the phase gradient of light. Size-dependent optical forces drive nanoparticles of different sizes in solution at different speeds, leading to their separation. They demonstrated the separation of silver and gold nanoparticles with diameters ranging from 70 to 150 nm, achieving resolutions as low as 10 nm. Particle separation occurs under static flow conditions (Figure 10A,B).B). Shilkin et al.163applied optical forces to spherical silicon nanoparticles using high-quality Mie resonance for size-based nanoparticle separation. They could resolve nanoparticles with diameters of 130, 150, and 160 nm, achieving a resolution of 10 nm. Separation also occurs under static flow conditions (Figure 10C,D).D). To increase throughput, Wu et al.164combined optical and hydrodynamic forces to separate gold nanoparticles in a flowing system (Figure 10E,F).F). They demonstrated the separation of gold nanoparticles with diameters of 50 vs 100 nm and 100 vs 200 nm. The sorting purity for the 50/100 nm combination was 92%, while for the 100/200 nm group, it was 86%, with a throughput of 300 particles/min. The throughput was significantly higher than that under static conditions. They also reported successful nanoparticle separations with smaller heterogeneity (i.e., 50 nm and 70 nm). In addition to processing “hard” nanoparticles (e.g., Ag and Au), researchers have also attempted to classify “soft” particles with stiffness ranging from 10−10 to 10−8 N/m (e.g., polymers, viruses, and DNA). Shi et al.165simultaneously applied optical forces and drag to separate 100 and 150 nm polystyrene nanoparticles with single-nanometer precision.
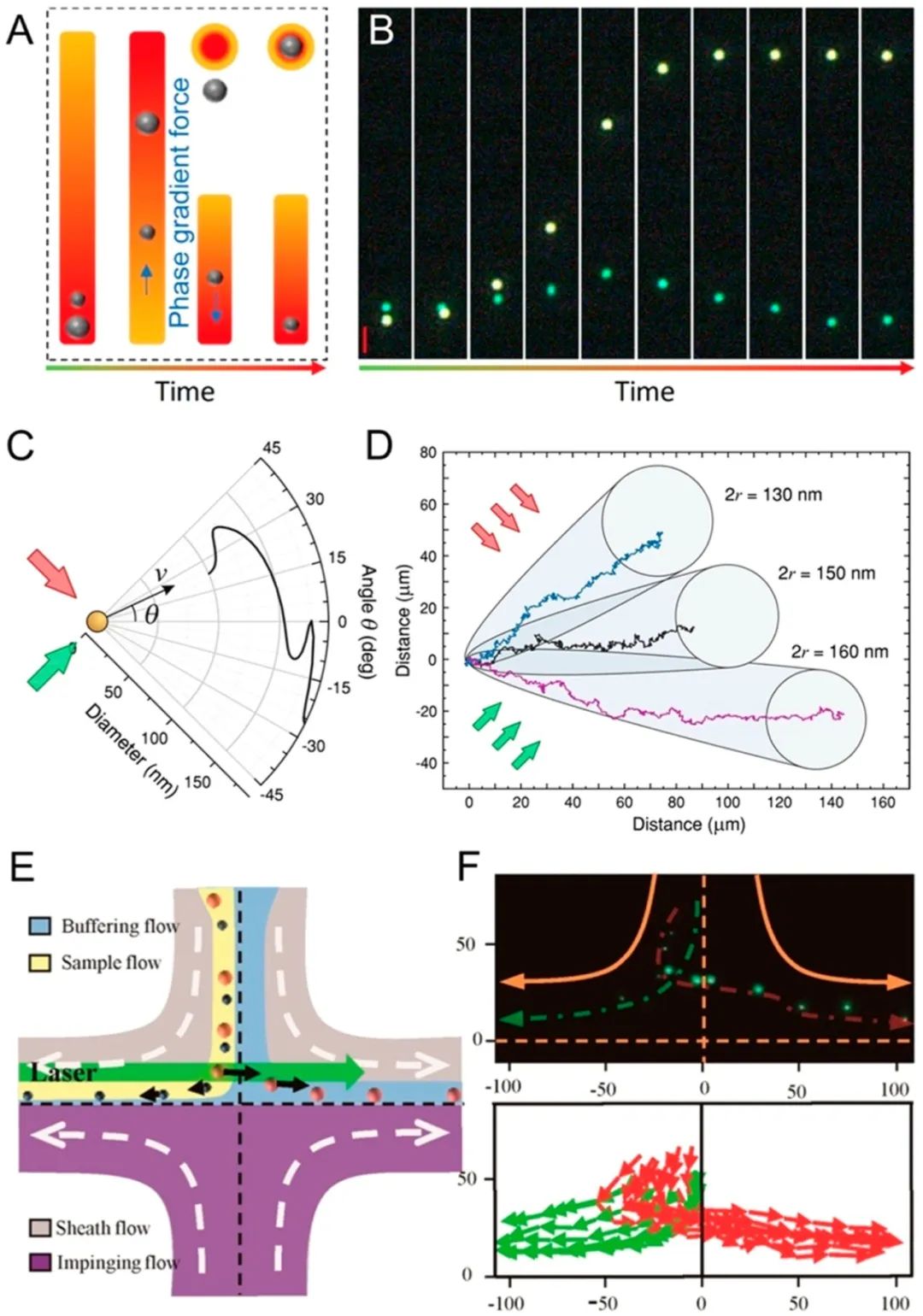
Figure 10.
Optical nanoparticle separation. (A, B) Separation of nanoparticles with the light phase gradient. Reproduced from ref162. Copyright 2018 American Chemical Society. (C, D) Separation of nanoparticles with Mie resonance. Reproduced from ref163. Copyright 2017 American Chemical Society. (E, F) Separation of nanoparticles combining optical and hydrodynamic forces. Reproduced from ref164. Copyright 2016 American Chemical Society.
Optical methods have also been used to enrich nanoparticles, typically achieved through laser-induced thermophoresis.166-169For example, Weinert et al.170combined laser-induced bidirectional flow with vertical thermophoretic molecular drift to concentrate biological nanoparticles (Figure 11A,B).B). They demonstrated the accumulation of 100-fold of 5-base DNA and 40 nm polystyrene nanoparticles within seconds. Later, Yu et al.171reported a laser thermophoresis-based DNA detection method (Figure 11C,D).D). They concentrated DNA-functionalized gold nanoparticles and fluorescent DNA probes to capture target DNA in free solution. Once DNA and probes bind, the thermophoretic properties of the fluorescent probes change. Their work revealed the detection of DNA in serum buffer without any channels, pumps, or washing steps. The thermophoretic effect can be more precisely controlled through plasmonic structures.172-174For example, Braun et al.175produced nanostructures by depositing gold films on glass slides, with the gold films acting as microscopic heat sources to create localized temperature gradients. They were able to capture and enrich 200 nm nanoparticles through localized thermophoresis at predetermined locations.
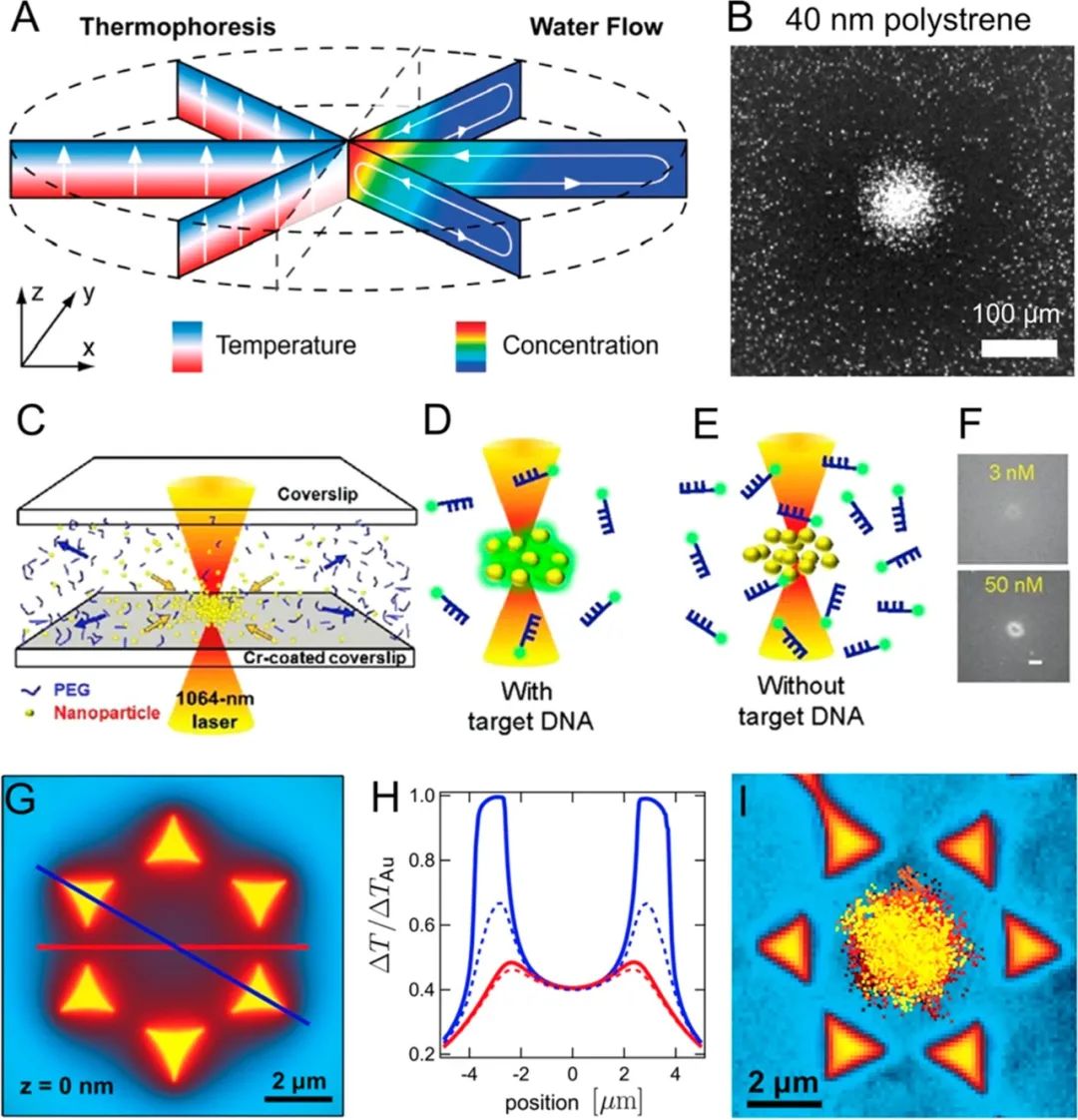
Figure 11.
Optical methods for enriching nanoparticles. (A) Mechanism of thermophoresis. (B) Its application in concentrating 40 nm polystyrene nanoparticles. Reproduced from ref170. Copyright 2009 American Chemical Society. (C, D) Using laser-induced thermophoretic effects to enrich DNA with probes for DNA detection. Reproduced from ref171. Copyright 2015 American Chemical Society. (E, F) Plasmonic structures modulating local temperature distribution. (G, H) Integrated plasmonic structures for concentrating nanoparticles at predetermined locations through thermophoretic effects. Reproduced from ref175. Copyright 2013 American Chemical Society.
Optofluidic methods are particularly suitable for manipulating nanoparticles. First, the interaction of light with nanoparticles provides a wide range of forces to drive the motion of nanoparticles. These forces include but are not limited to scattering forces and gradient forces, surface plasmon resonance, and thermofluid dynamics. Second, light is highly directional and can be precisely controlled by adjusting wavelength, power, and duration, allowing for high-resolution nanoparticle separation. Reported optical methods can separate nanoparticles with a resolution of 10 nanometers, which is challenging to achieve through other mechanisms. Third, optical methods are convenient to integrate into microfluidic devices. Nevertheless, optical nanoparticle separation also faces several theoretical and practical challenges: (1) Due to the various ways light can interact with nanoparticles, it is difficult to predict the separation performance for each nanoparticle. For example, the studies discussed earlier are based on single material systems (e.g., Ag or Au); however, once the material changes, the separation performance may differ significantly. (2) Most recent reports are based on metallic or silicon particles. When separating biological nanoparticles, biocompatibility is a concern, as the medium may absorb the energy of light and convert it into heat.
Electrophoresis
Nano-sized particles with different charges can be easily separated and enriched through microfluidic electrophoresis.176–180The mechanism of microfluidic electrophoresis is similar to conventional gel electrophoresis181,182or capillary electrophoresis,183which is based on the differences in solute electrophoretic mobilities (e.g., zeta potential176and size). Electrophoresis has been widely used for the separation of inorganic nanoparticles, proteins, peptides, and DNA.184,185Using microfluidic devices allows for greater flexibility in spatial configuration of the electric fields and sample flow. Sun et al.186used free-flowing microfluidic electrophoresis chips to separate a mixture of FITC-BSA, FITC-lysozyme, and FITC-pepsin based on charge and/or size. Jeon et al.187separated BODIPY molecular dyes2−and PTS4−based on charge differences through microfluidic electrophoresis. Interestingly, electrophoresis methods have also been used to separate nanoparticles of different shapes. For example, Hanauer et al.188demonstrated the separation of gold and silver nanoparticles based on size and shape through agarose gel electrophoresis after coating the nanoparticles with charged polymer layers. They verified the separation effect using color (the shape-dependent optical properties of gold and silver nanoparticles). They also showcased the separation of silver rods with length-to-width ratios of 8.3 ± 0.8 vs 3.1 ± 0.7, demonstrating shape-related separation capabilities.
Electrophoresis methods have been widely used for nanoparticle separation. They have several advantages. First, the electrophoretic force does not decrease cubically with particle size (it is linearly related to particle size);187,189therefore, electrophoresis-based methods can maintain high performance even at the nanoscale. Second, they can conveniently separate peptides and proteins. Since the charge of peptides and proteins often varies with pH, isoelectric focusing190,191can be used to move molecules in the presence of pH gradients until the net charge of the molecules is zero (i.e., isoelectric point). Third, electrophoresis methods can be used to separate surface-modified nanoparticles. Surface modification of nanoparticles is a key step in adjusting surface properties, which is crucial for catalysis and biomedicine.192–194As an example of separating nanoparticles with different surface properties, Wang et al.195utilized capillary electrophoresis to monitor changes in surface ligands of quantum dots. Integrating electrophoretic separation into microfluidic devices allows for more precise control of flow rates and electrodes in batch operations. On the other hand, microfluidic electrophoresis generates Joule heating112and may lead to bubble formation,196both of which can damage biological samples and hinder the consistency of device performance. Researchers have developed flow-induced electrophoresis,187buffer additives,197and thermal walls198to circumvent these limitations.
Microfluidic Affinity Isolation
Certain biological nanoparticles (e.g., viruses and exosomes) can be separated based on specific antigens expressed on their surface using targeted antibodies. Microfluidic affinity-based nanoparticle separation has been widely studied to enrich extracellular vesicles from various biological matrices. Chen et al.199reported a microfluidic exosome capture device that employed anti-CD63 modified microfluidic channels. A herringbone structure was fabricated on the ceiling of the microchannel to enhance capture efficiency. Compared to traditional ultracentrifugation, the microfluidic affinity capture method shortened sample processing time without the need for expensive instruments. Kanwar et al.200fabricated an exosome capture device with a circular wide channel that connects with a narrow fluid channel to facilitate interactions between exosomes and surface-immobilized antibodies (Figure 12A,B).B). Fluorescent microscopy was used to examine exosomes captured by the device or recovered for off-chip RNA analysis. Lo et al.201used microcolumn structures to enhance the capture of exosomes in microfluidic channels. Using biotinylated antibodies as capture antibodies, they were able to release exosomes after capture. Besides channel geometry, the surface properties of the channels are also crucial for the performance of affinity-based separations. Zhang et al.202developed a method to form nanostructures on channel surfaces by coating with graphene oxide and polydopamine (Figure 12C). This coating improved exosome capture while reducing nonspecific binding. Exosome ELISA assays based on this strategy achieved a detection limit of 50 μL−1 with a dynamic range of 4 orders of magnitude. To further enhance the detection sensitivity of exosomes, combining herringbone structures with nanoscale features on channel surfaces has proven effective.203,204Recently, Zhang et al. fabricated nanostructures on herringbone structures through self-assembly processes, achieving a detection limit of 10 exosomes/μL.204Moreover, affinity separation devices are often suitable for integrating the detection of functional units. He et al.205reported an integrated exosome separation and vesicular protein analysis based on two-stage immunomagnetic capture. The first stage used antibody-coated magnetic beads to separate exosomes. After on-chip lysis, the second stage of immunomagnetic separation allowed for the separation of target vesicular proteins for ELISA analysis. Im et al.206combined surface plasmon resonance with affinity capture to achieve multiplex detection of a set of protein biomarkers in exosomes. Jeong et al.207developed a portable integrated exosome separation and detection device by combining immunomagnetic capture and electrochemical detection for studying exosomes in plasma samples from ovarian cancer patients (Figure 12D). Besides exosomes, Wang et al.208developed a microfluidic affinity capture device that used anti-gp120 modified channel surfaces to separate HIV subtypes. The capture efficiency reached 75% for spiked human blood samples.
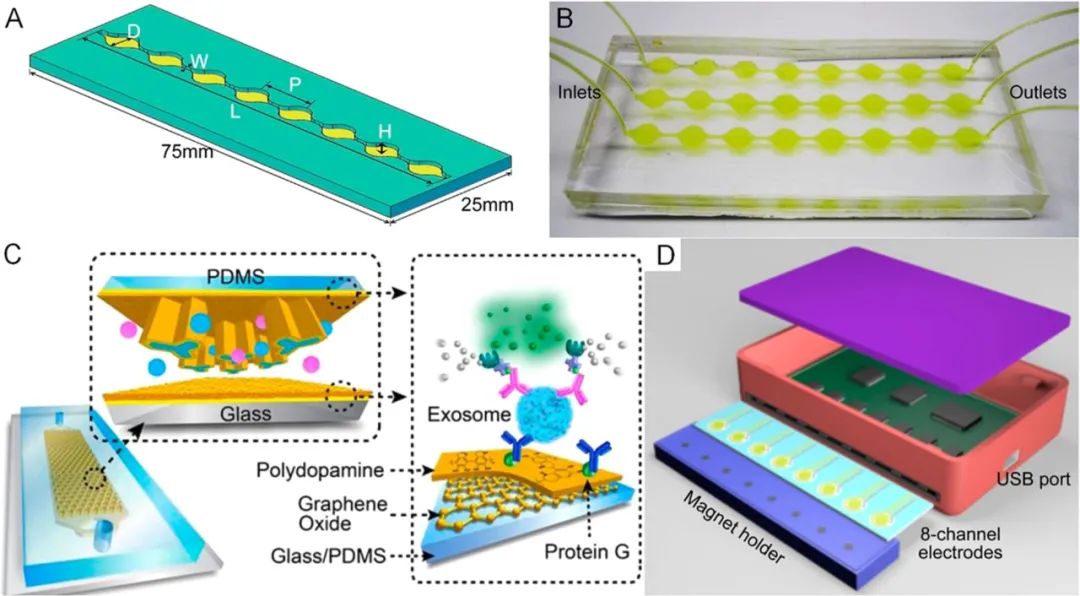
Figure 12.
Affinity-based nanoparticle separation. (A) Design of a circular microfluidic channel for enhanced exosome capture and (B) device image. Reproduced with permission from ref 200. Copyright 2014 Royal Society of Chemistry. (C) Improving exosome capture using graphene oxide and polydopamine surface coatings. Reproduced with permission from ref 202. Copyright 2016 Royal Society of Chemistry. (D) Integrated exosome separation detection device based on immunomagnetic capture and electrochemical detection. Reproduced from ref 207. Copyright 2016 American Chemical Society.
Affinity-based separation enables researchers to isolate phenotypically pure biological nanoparticles from mixtures of background particles, providing more relevant results for biologists compared to physical property-based methods. Traditionally, affinity separations typically employ antibody-labeled magnetic particles or affinity chromatography. To date, many microfluidic affinity separation methods have been reported to have advantages over traditional methods. First, microfluidics can precisely control fluid distribution and shear stress to achieve optimal separation conditions, maximizing capture efficiency while maintaining low levels of nonspecific binding. Second, microfluidic devices have a larger surface area-to-volume ratio, facilitating interactions between particles and affinity surfaces, thereby enhancing capture efficiency. Third, affinity separation microdevices can be integrated with upstream sample preparation units and downstream particle characterization units to streamline the entire nanoparticle separation workflow. Nevertheless, designing affinity separations requires prior knowledge of the biochemical characteristics of the nanoparticle surfaces.
Comparison Between Bulk and Microfluidic Methods for Nanoparticle Separation and Enrichment
Nano-sized particles can be separated and enriched through bulk methods capable of handling large sample materials. Applications such as water treatment209,210utilize bulk ultrafiltration114,211-213and reverse osmosis214,215technologies to remove nanoparticles (e.g., ions and proteins) and microparticles (e.g., bacteria and particulates). Filtration216-218centrifugation219-222electrophoresis52,223–225 and chromatography226-229are mature bulk methods for separating proteins and cellular components from liquid media and/or other micro/nano-scale objects. Although bulk methods can be robust when processing large-volume samples, they can also have limitations in certain applications. First, bulk methods often require a minimal sample volume to perform nanoparticle separation; however, obtaining such volumes from rare samples may be problematic in diagnostics or catalysis. Second, bulk methods struggle to maintain uniform separation conditions throughout the separation unit. For instance, the so-called “smile effect” occurs in protein/DNA gel electrophoresis due to differences in electrophoretic mobilities of ions at the center and edges of gel plates caused by uneven temperatures, thereby impairing separation performance.230Considering the small size of nanoparticles and the slight differences between nanoparticle subpopulations, uneven separation conditions hinder achieving high separation efficiency. Therefore, while bulk methods are robust and mature, there is a significant need for smaller, more precise nanoparticle separation and enrichment methods.
To address these limitations, microfluidic devices45,46,231-234can be effective.235As the size of separation units shrinks to the microscale, microfluidic technology offers several advantages over bulk methods. First, it is easier to maintain uniform force fields and separation conditions at smaller length scales, aiding in achieving higher separation performance (e.g., better purity and yield). Second, microfluidic technologies excel in handling nanoparticles contained within small sample volumes, making them particularly suitable for diagnostic and catalytic applications. Third, microfluidic technologies feature compact devices, making them easy to integrate into existing nanoparticle workflows. By eliminating the need to transfer samples between multiple units, process stability is improved, and batch-to-batch variability is reduced. Nevertheless, we recognize that microfluidics is not a one-size-fits-all solution for nanoparticle separation and enrichment. For instance, microfluidics shares the same theoretical limitations as bulk methods when dealing with small particles down to the nanoscale, and in certain applications, additional investments in devices and equipment are required.
Addressing Theoretical Challenges in Microfluidic Nanoparticle Separation and Enrichment
As discussed above, microfluidic methods have clear advantages over bulky benchtop methods in controlling separation and enrichment conditions, minimal sample volume requirements, and device compactness. However, these methods still face challenges both theoretically and experimentally. For example, a significant body of literature shows that size is currently the primary feature for nanoparticle separation and enrichment,236where nanoparticles are fractionated by their size and then enriched for particles of a certain size for subsequent analysis. This strategy has achieved great success when applied to microparticles and cells for the following reasons. First, size is the most direct feature that can be easily confirmed through microscopic imaging. Second, most driving forces used to deflect particles are proportional to the particle volume; thus, even slight differences in particle diameter can lead to significant differences in the forces applied to these particles. Third, size-based separations typically require minimal sample preprocessing (e.g., labeling), simplifying the separation process.
Despite the success of microfluidic separations and microparticle enrichment,237-242simply extrapolating size-based methods to nanoparticles presents some theoretical limitations. First, compared to the microscale, the driving forces at the nanoscale diminish rapidly. For example, the magnitude of acoustic radiation forces,68,72,243which drive nanoparticle deflection in acoustic isolation, is proportional to the particle volume; however, the resistance hindering particle motion is proportional to the particle diameter.243Therefore, deflecting 100 nm particles is much more challenging than 1 μm particles in laminar flow. Similar volumetric proportional forces also govern DEP-based isolation.235,244In addition to the weakened driving forces, Brownian motion noise245,246is non-negligible for nanoparticles. In summary, separation and enrichment at the nanoscale is theoretically more challenging than at the microscale. To overcome the performance decline of size-based separation and enrichment at the nanoscale, the following strategies are recommended.
Optimizing Parameters for Nanoscale Separation and Enrichment.
Researchers often attempt to extrapolate separation and enrichment parameters from the microscale to the nanoscale. For instance, they reduce pore sizes in filtration,125shorten wavelengths in acoustics,247,248increase power input, redesign channel shapes,132flow rates,143and apply recirculation249,250for repeated isolation. However, increasing the power of optical and acoustic methods requires additional power supplies and cooling systems. Recirculation effectively increases the path of particles under impact, enhancing purity but damaging yield. Although parameter optimization may present additional challenges, it is often one of the primary considerations for improving the performance of nanoparticle separation and enrichment methods.
Scaling Down Device Sizes.
In addition to the limitations imposed by the properties of the driving forces mentioned above, most microfluidic devices are still too large to manipulate nanoparticles. Thus, scaling down microfluidic devices to nanoscale can significantly enhance the performance of nanoparticle separation and enrichment. For example, DLD has shown outstanding performance in separating microscale cells and particles. To apply DLD in nanoparticle separation, researchers reduced the gap between each pillar and switched the device material from PDMS to silicon to create nano-DLDs capable of separating nanoparticles with diameters smaller than 100 nm.132In addition to nano-DLD, nanoscale channels have also been considered; Huh et al.251demonstrated tunable nanoscale channels capable of selectively screening particles with approximately 20 nm quantum dots. Stavis et al.252developed nanoscale fluid channels with a maximum depth of 620 nm, a minimum depth of 80 nm, and an average step size of 18 nm, sorting bimodal mixtures of nanoparticles through size exclusion at the nanoscale. They separated nanoparticles with diameters of 100 and 210 nm, claiming that the minimum difference in diameter could be as small as 18 nm. However, scaling down devices to the nanoscale requires alternative materials (e.g., switching from PDMS in micro-DLD to silicon in nano-DLD) and manufacturing techniques (e.g., transitioning from soft lithography to high-resolution lithography). Additionally, as the nanoscale range further expands, molecular-level interactions cannot be ignored, adding further complexity to particle manipulation.253-255
Dynamic Adjustment of Nanoparticle Sizes.
Zeming et al.133adjusted the Debye length of polystyrene microspheres in real-time by varying the NaCl ion concentration, thus dynamically adjusting the isolation effect of their DLD devices. Their strategy can be extended to various nanoparticles. For example, the size of biological nanoparticles (e.g., DNA and proteins) depends on the biochemical factors in the surrounding environment (e.g., pH and salt concentration).256Similarly, the hydrodynamic radius of many synthetic nanoparticles also depends on the surface interactions with molecules in the suspension medium.257Therefore, controlling the surrounding environment can increase their size or amplify size differences between two particles. However, this method directly alters the size and separation effects and requires additional post-separation procedures to restore nanoparticles to their native structures, which may affect the biological activity of proteins or DNA.
Developing Mechanisms Based on Different Chemical and Physical Properties.
Biological nanoparticles such as DNA, proteins, vesicles, and exosomes can be separated and enriched in one step using affinity-based methods.199,203,205-207In this case, the advantages and disadvantages of affinity-based methods, size-based methods, and other mechanisms must be considered. Compared to antibody-based operations, size-based operations lack labeling, immuno-binding, and washing steps but lack specificity. Besides size, shape-based mechanisms188,219,236may serve as a good alternative to size-based isolation. Shape is crucial for the catalytic applications of nanoparticles as it determines the surface area-to-volume ratio. Shape also plays an important role in the bioavailability of nanoparticles, with studies showing that cylindrical nanoparticles interact with cells very differently than spherical nanoparticles.258,259Moreover, characterizing shape differences is as straightforward as characterizing size. Nevertheless, understanding how different shapes respond to force fields remains unclear, hindering shape-based nanoparticle separation and enrichment. For example, particles with the same volume but different shapes seem to exhibit different acoustic fluid forces and DEP;188,219,236unfortunately, quantitative analysis of these forces is not straightforward.
Future Development and Commercialization Considerations for Microfluidic Nanoparticle Separation and Enrichment Systems
Despite the numerous demonstrations of microfluidic nanoparticle separation and enrichment, several aspects still need improvement, including application specificity, ease of integration, and accessibility for end-users.
Customizing Microfluidic Methods for Specific Applications.
Given the complexity of nanoparticles, it is unlikely that a single mechanism can be classified as a “one-size-fits-all” approach. For example, the separation method best suited for metallic nanoparticles may not be optimal for biological nanoparticles. In recent years, purifying and enriching extracellular vesicles from various biological fluids has become increasingly important. Targeting extracellular vesicles requires customization to accommodate the requirements and characteristics of the extracellular vesicles.76,132,144,147,227For example, certain applications require maintaining the integrity of extracellular vesicles, necessitating gentle separation mechanisms or mild separation procedures. Additionally, to facilitate the characterization of the protein or RNA content of vesicles,260–264one method needs to both enrich nanoparticles and demonstrate high compatibility with various biochemical characterization assays.
Developing Integrated Microfluidic Separation-Enrichment-Analysis Platforms.
One of the advantages of microfluidic methods is their ability to integrate with various working mechanisms or functional units. As an example of integrating different working mechanisms, acoustic and magnetic methods can be combined with surface affinity to separate and enrich exosomes.76Here, exosomes specifically bind to microparticles through surface biomarkers for enrichment; then, an acoustic or magnetic field is applied to deflect the bonded exosomes from free exosomes for separation. In addition to integrating multiple working mechanisms to enhance separation performance, combining nanoparticle separation, enrichment, and characterization units into a single system holds promise for providing “sample input-answer output” devices. For example, Cheng et al.107demonstrated the enrichment of Ag nanoparticles with bacteria to facilitate SERS detection; Yu et al.171demonstrated the enrichment of DNA with probe-coated nanoparticles for fluorescence detection; Yeo et al.104,109reported concentrating viruses onto nanoscale tips for fluorescence and electron microscopy imaging detection.
Improving Accessibility of Microfluidic Systems Through Commercialization.
Despite the promising prospects of microfluidic methods, ultracentrifugation remains the most widely used method for nanoparticle separation and enrichment, despite its biocompatibility issues. This is primarily because ultracentrifugation and other conventional methods are already on the market and have established operating protocols that can be performed even by non-instrument experts. Most existing microfluidic methods remain in the proof-of-concept stage and must be executed by microfluidic technicians.46Many non-standard device manufacturing and operation procedures make microfluidic methods difficult to adopt by other communities. Efforts should be made to improve device manufacturing procedures and reduce the need for peripheral equipment. For instance, core components of microfluidic channels can be made from paper materials,265,266plastics267,268or glass,43,55instead of PDMS, using standard industrial manufacturing processes. Ultimately, the broader impact of instruments comes from their commercialization. In this regard, one example is the commercially available Eclipse AF4, which utilizes flow-field-flow separation methods for size-based separation of proteins, liposomes, viruses, and other nanoparticles.119,121,123On the other hand, in resource-limited environments, developing on-demand nanoparticle separation, enrichment, and characterization systems based on microfluidic methods may be particularly appealing. For example, Bachman et al.82developed an on-demand acoustic flow pump and mixer by combining a smartphone, Bluetooth speaker, sharp-edged acoustic devices, and simple portable microscopes. They created a functional prototype using commercial Arduino components, which holds great potential for point-of-care applications.84
Emerging Markets for Microfluidic Nanoparticle Separation and Enrichment in Biomedicine
In this section, we provide insights into the biological and medical applications of microfluidic nanoparticle separation and enrichment. We will cover basic research on membrane-bound and non-membrane organelles, as well as clinical diagnostics and therapeutics.
Research on Membrane-Bound Organelles.
To date, numerous microfluidic methods have been reported for separating and enriching extracellular vesicles.76,132,144,147,227Besides extracellular vesicles, many membrane-bound organelles of significant importance to human health include mitochondria, secretory vesicles, endosomal and lysosomal systems, peroxisomes, lipid droplets, and autophagosomes.269These organelles are often nanoparticle-sized with various transmembrane markers for their vesicular transport and function.269To study the functions, development, and diagnostic uses of organelles, methods for separating and enriching these organelles are preferable. While these organelles are typically separated and enriched through ultracentrifugation, microfluidic methods are achievable targets for these organelles due to their nanoscale size and enriched surface markers. In fact, microfluidic methods are expected to have advantages over traditional ultracentrifugation, as the precipitation formed by high-speed rotation (>100,000 g in ultracentrifugation) can damage membrane-bound organelles and alter protein functions.
Research on Non-Membrane Organelles.
In addition to membrane-bound organelles, many cellular compartments are non-membranous (e.g., non-membranous organelles).271They lack lipid bilayer boundaries, often exist transiently, and are formed through liquid-liquid phase separation mechanisms.272Despite current biological research revealing the importance of non-membrane compartments in neurodegenerative diseases,273,274stress responses,275and many other important physiological processes,271reports on their separation and concentration are scarce due to technical challenges. For instance, stress granules,276which are non-membranous cellular compartments that appear when cells are under stress, consist of protein and RNA-rich aggregates with diameters of 100-200 nm. Recently, they have been separated and collected using multi-step immunoaffinity methods.277Non-membranous organelles seem unsuitable for centrifugation since they lack membrane structures, making them susceptible to degradation under strong centrifugal forces. In this regard, microfluidic methods excel in handling these organelles, as they can operate under gentle conditions, continuously separating “fresh” objects and processing small-volume samples.
Diagnostic Applications.
Nanoparticles have been extensively studied and widely applied in disease diagnostics. “Hard nanoparticles” (e.g., gold) conjugated with antibodies can bind and detect protein biomarkers secreted by tumor cells.278Superparamagnetic nanoparticles have been used as contrast agents to improve the resolution of magnetic resonance imaging.279On the other hand, “soft nanoparticles” (e.g., exosomes and extracellular vesicles) have been used as diagnostic biomarkers for cancer76and central nervous system diseases.280Separation and enrichment of nanoparticles can benefit diagnostics in several ways. First, when using “hard nanoparticles” as diagnostic probes, separation and enrichment procedures can enhance diagnostic performance, as the optical and magnetic responses are closely related to the size and shape of the nanoparticles. Second, the separation and enrichment of “soft nanoparticles” provide possibilities for disease diagnostics. Similar to exosomes, other membrane and non-membrane organelles separated and enriched through microfluidic methods can serve as biomarkers for diseases. In this regard, microfluidic methods are advantageous as they can handle small-volume samples that can be collected non-invasively. Using this approach, bedside microfluidic devices can be developed for early disease diagnosis and therapeutic monitoring.
From “Precision Medicine” to “Ultra-Precision Nanomedicine.”
Interactions between medical nanoparticles and biological systems depend on the properties of nanoparticles, including their size, shape, surface charge, surface roughness, and hydrophilicity/hydrophobicity.26,30,31,258,281These characteristics can affect the specificity and toxicity of nanodrugs. To achieve “precision medicine,” materials scientists have designed various strategies to enhance the selectivity of nanoparticles282,283to specific tissues or cell populations. Microfluidic researchers aim to enhance the uniformity of nanoparticles by increasing the separation and enrichment steps, thereby facilitating the manufacture of “ultra-precision nanomedicine.” For example, better uniformity in nanoparticle size can enhance their circulation time in the bloodstream and tumor targeting capabilities. It has been reported that nanoparticles with diameters of approximately 100 nm benefit from enhanced permeability and retention effects for blood circulation and tumor accumulation;284on the other hand, nanoparticles smaller than 10 nm can cause genetic toxicity through point mutations, chromosomal fragmentation, and DNA strand breaks.285In summary, impurities from small nanoparticles can lead to severe side effects during nanoparticle cancer therapy. However, nanoparticles produced through bottom-up (e.g., nucleation growth) or top-down mechanisms (e.g., ultrasound treatment and fracturing) often have a wide size distribution. Separation methods will become powerful tools for producing uniform nanoparticles, and enrichment methods will increase the concentration of target nanoparticles, thereby enhancing uniformity, reducing unnecessary side effects, and ultimately achieving ultra-precision nanomedicine.
Conclusion
In recent years, the number of microfluidic technologies for separating and enriching nanoparticles has rapidly increased. This article discusses various techniques, including acoustic flow, dielectrophoresis, microfluidic filtration, deterministic lateral displacement, inertial microfluidics, optofluidics, electrophoresis, and microfluidic affinity isolation. Since this review is aimed at readers from diverse backgrounds, we provide an overview explaining the potential mechanisms behind various microfluidic methods. The governing equations behind each method are well described in the following reviews on acoustic flow,56-61,63,67,72DEP,88,90,93,96,111inertial microfluidics,135,136,141and DLD,128,129among others.
By gaining a deeper understanding of the advantages and disadvantages of each technique, we hope to provide researchers with the necessary information to choose the most suitable microfluidic method for their research needs. Compared to traditional bulk methods, microfluidic methods offer numerous advantages, including the ability to maintain uniform separation conditions at smaller length scales for higher separation performance, as well as the capability to handle nanoparticles in small-volume samples. Moreover, microfluidic devices are compact, making them easy to integrate into existing nanoparticle workflows. In laboratory settings, both soft nanoparticles (e.g., DNA, exosomes, and viruses) and hard nanoparticles (e.g., metallic and silicon nanoparticles) have been comprehensively validated for the performance of microfluidic methods. In the near future, with increased commercialization efforts, we expect microfluidic technologies for nanoparticle separation and enrichment to have a broader impact in both research and commercial environments. By making microfluidic technologies more widely available and user-friendly for researchers from different backgrounds, numerous applications and research directions will be realized, such as studying the roles of non-membranous organelles in various diseases.
Vocabulary
| Nanoparticle Separation | A process that acts as a selective barrier, allowing one component in a mixture of nanoparticles to pass relatively freely while retaining or deflecting other components. |
| Nanoparticle Enrichment | A procedure performed to increase the concentration of specific nanoparticles for collection. |
| Soft Nanoparticles | Organically occurring nanoparticles, such as proteins, DNA, viruses, and exosomes. |
| Hard Nanoparticles | Synthetically produced inorganic nanoparticles, such as gold, silver, and silicon. |
| Acoustic Flow | A method that combines acoustic manipulation with microfluidic devices. |
| Optofluidics | A method that combines optical operations with microfluidic devices. |