
1. Overview of Biochemical Diagnostic Instruments
The biochemical analyzer, also known as a biochemical instrument, is a device that uses photometric colorimetry and biochemical analysis methods to measure specific chemical components in body fluids. Due to its fast measurement speed, high accuracy, and low reagent consumption, it has been widely used in hospitals, epidemic prevention stations, and family planning service stations at all levels.
The fully automated biochemical analyzer is one of the most commonly used important analytical instruments in clinical testing. It is mainly used to determine various biochemical indicators in serum, plasma, or other body fluids, such as glucose, albumin, total protein, cholesterol, and transaminases, which are of significant importance in auxiliary diagnosis, efficacy testing, health checks, and drug abuse detection. It is an essential clinical testing device for medical and disease control units at all levels and is also a commonly used instrument in epidemic prevention, quarantine, and biological research. Its clinical application range is expanding, making it one of the fastest-growing fields in the global medical device industry.
Biochemical analysis is one of the important means of modern medical clinical diagnosis and disease prevention. The automatic biochemical analyzer can regularly and continuously monitor various reaction types of analytical measurements. Besides general biochemical project determinations, it can also measure special compounds such as hormones, immunoglobulins, and blood drug concentrations. By integrating the biochemical analysis results of blood and other body fluids with other clinical data, it provides a basis for diagnosing diseases and appropriately evaluating organ functions. It can also identify complicating factors and determine future treatment benchmarks and measures.
In contemporary hospitals, the automatic biochemical analyzer is one of the basic and indispensable medical devices for diagnosing diseases, monitoring conditions, observing treatment effects, making prognoses, and prevention. The diagnosis of acute pancreatitis, diabetic ketoacidosis, myocardial infarction, uremia, acid-base balance disorders caused by dehydration or edema, and electrolyte disturbances cannot be separated from biochemical analyzers; tests for liver function, kidney function, protein balance, lipid metabolism, glucose metabolism, potassium, sodium, chloride, calcium, phosphorus, magnesium, and trace elements are all conducted by biochemical analyzers. The results obtained from biochemical analyzers affect the medical quality across the entire hospital and are critical to patient safety.
2. Principles of Biochemical Diagnostic Instruments
The automatic biochemical analyzer is a high-tech product that integrates optics, machinery, electricity, and fluid. It can be roughly divided into four parts: the sample introduction system, optical system, control system, and data processing system. The sample introduction system is the premise of analysis, the optical system is the core of the entire instrument, the control system ensures the analysis, and the data processing system expands functionality. Currently, the vast majority of biochemical analyzers operate based on photometric colorimetry principles, and their structure can be roughly seen as composed of a photometric colorimeter or spectrophotometer and a microcomputer.
In terms of structural composition, the biochemical analyzer consists of a sample holder, sampling device, reaction cell or reaction pipeline, incubator, detector, microprocessor, printer, and functional monitor, among others. Its specific working principle is as follows: a monochromatic light beam illuminates the colored liquid in the colorimetric cell, and the absorption of light energy by the sample being tested is detected by converting the light signal into a corresponding electrical signal. This signal is amplified, rectified, and converted into a digital signal, which is sent to the computer. At the same time, the computer controls the power to drive the filter wheel and sample tray. The computer processes, calculates, analyzes, saves the measurement data based on the user-selected working mode, and the printer simultaneously prints the corresponding results. Finally, after measuring each group of samples, the colorimetric cell is cleaned.
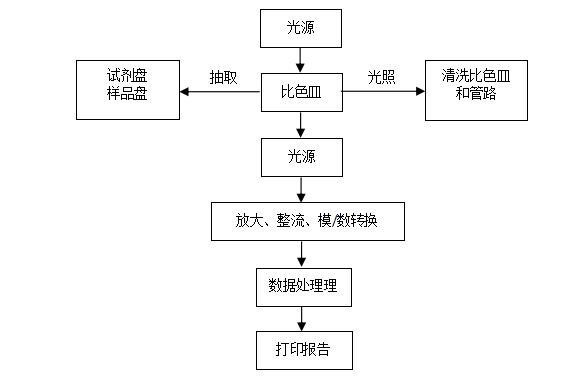
Working Principle of Biochemical Analyzers
3. Classification of Biochemical Diagnostic Instruments
Since Technicon Company in the United States successfully produced the world’s first fully automated biochemical analyzer in 1957, various types and functions of fully automated biochemical analyzers have emerged, marking a significant step towards automating clinical biochemical testing in hospitals. To date, the development of biochemical analyzers has been rapid, and the classification methods are rich and varied. Generally, they can be classified in the following ways.
01
Classification by Degree of Automation
1. Semi-Automatic Biochemical Analyzers
In the analysis process, some operations need to be performed manually (such as sample addition, incubation, colorimetric suction, result recording, etc.), while other operations can be completed automatically by the instrument. Such instruments are referred to as semi-automatic biochemical analyzers. Their characteristics are small size, simple structure, high flexibility, and low price.
2. Fully Automatic Biochemical Analyzers
The entire process from sample addition to outputting test results is completely automated by the instrument. The operator only needs to place the sample in a designated position on the analyzer, select the program, and start the instrument to wait for the test report, with no manual intervention required during the process. Since there are no manual operation steps during analysis, subjective errors are minimal, and due to the automatic reporting of abnormal conditions and automatic correction of its working state, the systematic error is also small.
02
Classification by Structure and Principle
1. Continuous Flow (Pipeline) Analyzers
Continuous flow analyzers refer to instruments where the chemical reaction of the same testing items is completed in the same pipeline after mixing the samples with reagents. Such instruments can generally be divided into air-segmented systems and non-segmented systems. The air-segmented system means that a small segment of air separates each sample, reagent, and the mixed reaction liquid in the suction pipeline, while the non-segmented system uses reagent blanks or buffer solutions to separate the reaction liquids of each sample. Among pipeline analyzers, the air-segmented system is the most common.
2. Discrete Analyzers
Discrete analyzers are programmed in a way that requires manual operations and uses rhythmic mechanical operations to replace manual work, with each stage connected by conveyor belts and operated in sequence. The chemical reactions of each sample mixed with reagents are completed in their respective reaction cups.
3. Centrifugal Analyzers
Centrifugal analyzers refer to instruments where each sample is mixed with reagents under the action of centrifugal force in their respective reaction chambers, completing the chemical reaction and measurement almost simultaneously, resulting in high analytical efficiency.
4. Dry Slide Analyzers
Dry slide analyzers refer to instruments where reagents are solidified on carriers such as film or filter paper, and each sample is added to the corresponding test strip for reaction and measurement. Their advantages include fast operation and portability, currently used mainly in emergency and field tests.
5. Bag-type Analyzers
Bag-type analyzers refer to instruments that use reagent bags instead of reaction cups and colorimetric cups, where each sample reacts and is measured in its reagent bag.
03
Classification by Compatibility of Reagents and Instruments
1. Closed Systems
The instrument and reagents are bundled for sale and use, generally with reagent brands specified by the instrument manufacturer, and other manufacturers’ reagents cannot be used.
2. Open Systems
The instrument and reagents are sold and used separately, with no reagent brands specified by the instrument manufacturer, allowing users to choose freely.
04
Classification by International Testing Practices
1. Small Instruments
Small instruments are generally single-channel and have slower testing speeds.
2. Medium Instruments
Medium instruments are generally multi-channel and can test 2-10 items simultaneously, with some instruments having fixed testing items while others allow for arbitrary selection.
3. Large Instruments
Large instruments are generally multi-channel and can test more than 10 items simultaneously, with free selection of analysis items.
05
Classification by Testing Channels
1. Single-channel Biochemical Analyzers
Single-channel biochemical analyzers can only test one item at a time, but the items can be changed, and the testing speed is slower.
2. Multi-channel Biochemical Analyzers
Multi-channel biochemical analyzers can simultaneously test multiple items at a time.
4. Development History of Biochemical Diagnostic Instruments
The biochemical analyzer has gone through three stages, from the initial spectrophotometer to semi-automatic biochemical analyzers, and now to the widely used fully automatic biochemical analyzers.
First Generation: Spectrophotometer, which uses ultraviolet light, visible light, infrared light, and laser light to measure the absorption spectrum of substances, a method known as spectrophotometry or spectrophotometric technology, using an instrument called a spectrophotometer. The advantages of spectrophotometer detection are direct reading of absorbance, simple operation, and low cost; its disadvantages are that it cannot directly calculate concentration values, has large errors, and fewer detectable items.
Second Generation: Semi-automatic biochemical analyzers, which require some operations (such as sample addition, incubation, colorimetric suction, result recording, etc.) to be performed manually during the analysis process, while other operations can be completed automatically by the instrument. The characteristics of these instruments are small size, simple structure, and high flexibility, allowing them to be used separately or in conjunction with other instruments, and they are inexpensive.
Third Generation: Fully automatic analyzers, where the entire process from sample addition to outputting results is completely automated. The operator only needs to place the sample in a designated position on the analyzer, select the program, and start the instrument to wait for the test report. Fully automatic biochemical analyzers have more reagent and sample positions than semi-automatic ones, faster testing speeds, and eliminate errors caused by different manual reagent addition techniques, resulting in better accuracy and repeatability.
01
International Development History of Biochemical Analyzers
In the early 19th century, the most primitive manual methods were used to complete a very limited number of biochemical indicator tests. During this stage, the methods for loading samples, reagents, and other liquids mainly relied on pipettes, which had extremely low work efficiency and high errors. In the 1950s, with the advent of automatic diluters, the loading of liquids achieved automation or semi-automation, and semi-automatic colorimeters were successively used, completing sample colorimetric determinations using fixed flow colorimetric cups.
In 1957, Technicon Company in the United States manufactured the world’s first biochemical analyzer based on Professor Skeggs’ design, leading to the emergence of various models and functions of fully automatic biochemical analyzers, developing rapidly. In 1965, the discrete automatic analyzer was born, which operates similarly to manual operations, with samples in separate reaction cups.
The 1970s opened up reflective light analyzers paired with dry chemical reagents, improving the accuracy, precision, multifunctionality, and analysis speed of biochemical analysis. The application of analytical technologies such as spectrometry, centrifugation, chromatography, electrophoresis, radioactive isotopes, and immunological techniques has significantly promoted the rapid development of various clinical biochemical testing instruments. In the early 1980s, TECHNICON Company in the United States invented a selectable measurement method for instruments that could overcome cross-contamination between samples, raising the level of automatic biochemical analyzers to a new height. In the late 1980s, dry chemical analyzers using solid-phase enzymes, ion-specific electrodes, and multi-layer membranes were developed. Since the 1990s, with technological advancements, the technology of biochemical analyzers has been developing towards various functional improvements, with an increasing number of detectable items and higher accuracy and precision in analysis, better meeting the diverse needs in laboratory management.
02
Development History of Biochemical Analyzers in China
In the mid-1970s, institutions such as the Shanghai Medical Device Research Institute, Beijing Medical Instrument Research Institute, Beijing Analytical Instrument Factory, and Beijing Biochemical Instrument Factory developed continuous flow, discrete, and centrifugal biochemical analyzers. Although domestic biochemical analyzers with independent intellectual property rights were developed at that time, their quality was poor and unsatisfactory. Even those that were prototyped were not marketable, and most of the instruments used domestically still depended on imports.
In 1972, the Italian Embassy in China donated a continuous flow automatic biochemical analyzer to Peking Union Medical College Hospital; in 1975, Tianjin Medical University Second Hospital introduced a two-channel continuous flow automatic biochemical analyzer from Italian company Carlo Erba. Based on the study of these two instruments, Beijing Biochemical Instrument Factory launched a three-channel continuous flow automatic biochemical analyzer, which won a national science and technology conference award in 1978, but unfortunately did not have market effects.
During the widespread use of automatic biochemical analyzers in clinical chemistry laboratories in China, a significant influence was made by the introduction of thousands of MicroLab semi-automatic biochemical analyzers by the Dutch company Vital around 1986. To bridge the gap between domestic clinical laboratories and developed countries in terms of automation, developing fully automatic biochemical analyzers became the preferred topic in domestic testing medicine at that time.
Since the 1990s, through persistent efforts, various models of semi-automatic and fully automatic biochemical analyzers have been independently developed domestically. The development of automation in clinical chemical testing in China can be summarized in seven periods, as shown in the following figure.
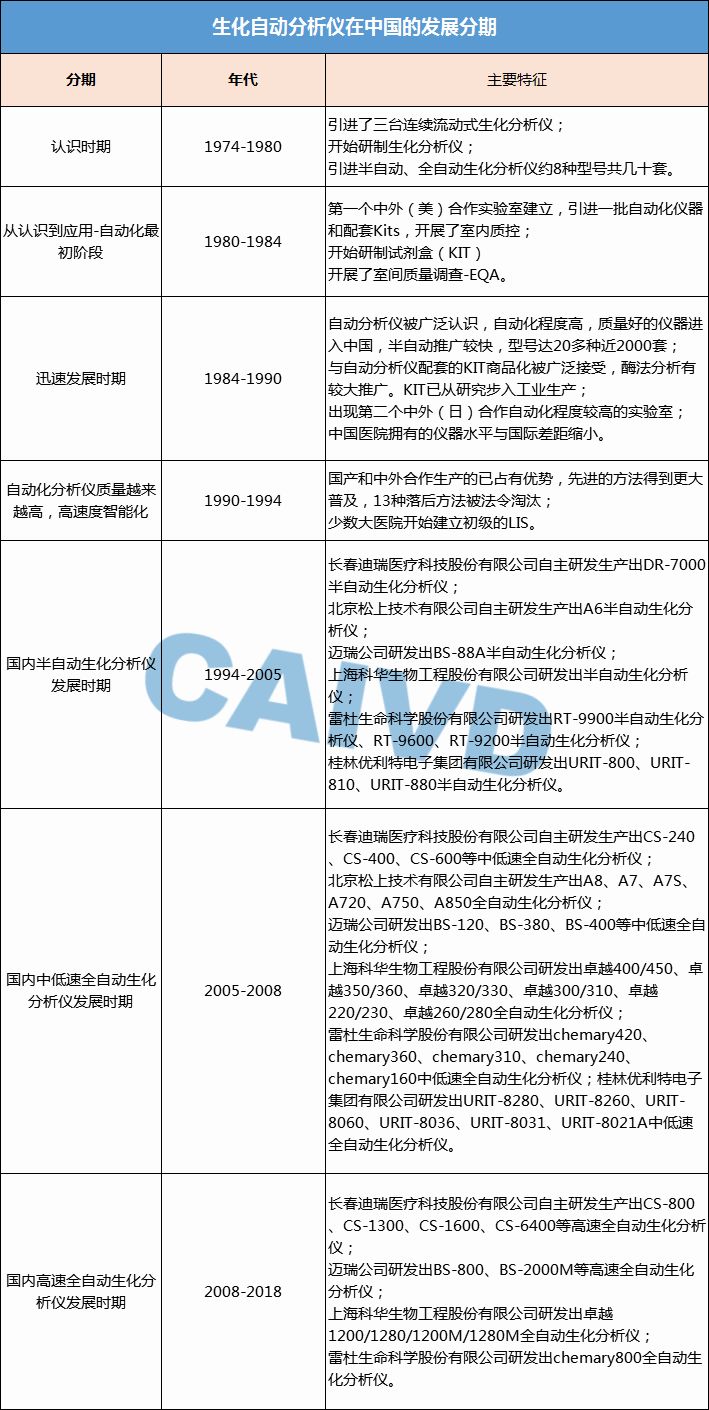
5. Main Circulating Products in the Biochemical Diagnostic Instrument Market
With the continuous development of fully automatic biochemical analyzers and the increasing speed of testing, we classify fully automatic biochemical analyzers according to testing speed, mainly into the following speed categories: ≤300T/H, 300-400T/H, 400-600T/H, 600-800T/H, and >800 T/H.
6. Market Capacity of Biochemical Diagnostic Instruments
According to industry data and market sales data statistics from various subdivided companies, the biochemical market capacity was approximately 10.3 billion RMB in 2016, about 11.3 billion RMB in 2017, with a growth rate of about 9%. In the domestic IVD segment, the traditional biochemical market share is gradually declining, and the growth rate is gradually decreasing. As domestic biochemical testing matures and stabilizes, the subsequent market growth rate will gradually slow down. Imported brands are mainly dominated by Roche and Beckman, accounting for about a quarter of the market, while most others are domestic brands. The expected market growth in the biochemical sector for 2017 is around 10%, and the entire market has transformed into a blue ocean market, with most brands experiencing sluggish growth for various reasons.
Looking at the domestic market, the domestic replacement rate of biochemical products is close to 50%. Many manufacturers are present, and according to NMPA registration statistics, there are more than 200 companies related to clinical biochemistry in China. However, imported manufacturers still dominate high-quality clients such as large tertiary hospitals for a long time. Most fully automatic biochemical analyzers in tertiary hospitals are basically monopolized by foreign companies. This is mainly because it involves comprehensive technologies across optics, machinery, electricity, software, fluid paths, temperature control, and biochemical analysis, with complex system structures, strict control timing requirements, and high reliability and accuracy requirements. With the successful development of domestic biochemical diagnostic reagents and instruments, domestic biochemical analyzers have rapidly been promoted in hospitals below the tertiary level. Currently, some quality domestic manufacturers have gradually penetrated into the high-end domestic market of tertiary hospitals, such as Mindray, Kehua, and Dirui.
Foreign fully automatic biochemical analyzers have become very mature in technology after years of development. Companies such as Roche, Beckman, Abbott, Siemens, Johnson & Johnson, and Hitachi have launched high-performance, high-speed, modular biochemical instruments that can be linked with immunological testing. Domestic biochemical analyzers started later and have a weak technological foundation. Currently, domestic companies represented by Mindray, Kehua, and Dirui have successfully produced biochemical instruments that can compete with foreign companies, mainly targeting the mid-to-low-end market; while domestic companies such as Innohua, Jinrui, Pukang, and Tekang focus on the low-end market. From the market situation, large hospitals and laboratories generally prioritize foreign brands of fully automatic biochemical analyzers due to their higher requirements for technical parameters, performance, and brands. Small and medium-sized hospitals, due to smaller sample volumes and cost considerations, generally opt for medium and small biochemical analyzers. Thus, it can be seen that the high-end market in China is still dominated by foreign brands. As foreign brand product lines penetrate deeper, competition in the small and medium-sized hospital market will become more intense.
—  —
—

In Vitro Diagnostic New Media & Service Platform
Satisfy Curiosity | Solve Problems

Long press to followVideo account
Delivering FreshColorfulInformation

Long press to recognize the QR code
AddCustomer Service WeChatInvite you to join the group
Hotline: 4008-0571-82
Email: [email protected]
The articles transferred represent the original author’s views. If there is any infringement, please contact for deletion!
Click “View” to give me a little orange flower
