Meige’s syndrome (also translated as “Meige’s syndrome”) is also known as idiopathic blepharospasm-oromandibular dystonia syndrome. It is a rare type of segmental dystonia, belonging to a form of adult attention deficit hyperactivity disorder, characterized by blepharospasm, difficulty in opening the eyes, with or without involuntary movements of the mouth and mandible.
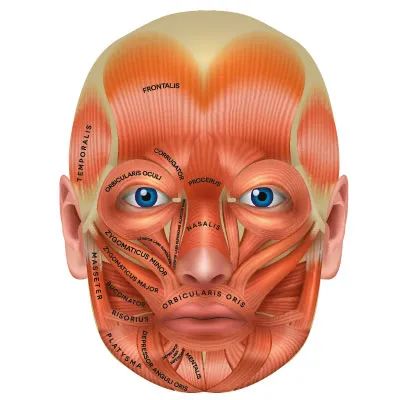
This syndrome manifests as involuntary blepharospasm. This permanent contraction is caused by the orbicularis oculi muscle.
Meige’s syndrome (MS) is a cranial dystonia characterized by involvement of the upper and lower cranial structures, including bilateral blepharospasm (BSP) and involuntary movements of the jaw muscles (oromandibular dystonia; OMD). The etiology and pathogenesis of this extrapyramidal disease are still unclear. Neurological and ophthalmological examinations often reveal no abnormalities, making diagnosis difficult and often leading to misdiagnosis. A small percentage of patients have a family history, but pathogenic genes have not yet been identified, and there is no cure, although treatment with botulinum toxin type A has effectively alleviated symptoms, and deep brain stimulation is increasingly being considered as a viable alternative treatment option.

The contraction between the muscles of the jaw and oral cavity is referred to as oromandibular dystonia. Meige’s syndrome (also known as blepharospasm-oromandibular dystonia, idiopathic facial movement disorder, or Brueghel syndrome) is a combination of oromandibular dystonia and primary blepharospasm. It is based on erroneous control of certain brain structures, which triggers involuntary blepharospasm.
Blepharospasm (BSP), oromandibular dystonia (OMD), and Meige’s syndrome (MS) are different but closely related movement disorders. MS is characterized by involvement of the upper and lower cranial structures, including involuntary movements of BSP and OMD. Most researchers and clinicians agree that MS, BSP, and OMD are not a single entity but a clinical syndrome of multifactorial origin. Patients with atypical Parkinson’s disease often exhibit BSP symptoms, and some patients with Huntington’s disease have OMD. The etiology of BSP, OMD, and MS remains elusive, but compelling evidence suggests that these diseases are multifactorial, involving one or more unknown genes, as well as epigenetic and environmental factors that converge to reach the disease threshold. Previously, dystonia was classified into primary (where dystonia is the only clinical symptom, including idiopathic or genetic diseases without neuropathological abnormalities) or secondary (dystonia caused by neurodegenerative diseases, acquired causes such as brain lesions, or hereditary diseases with progressive courses). A consensus update in 2013 on the phenomenology and classification of dystonia focused on clinical features and classified dystonia into isolated (where dystonia is the only clinical feature expressed, with no other neurological or systemic signs) or combined (where dystonic abnormalities are combined with other neurological or systemic signs).

Anatomical distribution classification of dystonia
In 1984, a special committee of the Dystonia Medical Research Foundation established a widely accepted definition of dystonia and a classification of dystonic movements. Dystonia is defined as a movement syndrome characterized by sustained muscle contractions, often resulting in twisting and repetitive movements or abnormal postures. It is noted that dystonia is typically triggered by action, and almost all dystonic movements have a directional tendency, which is usually sustained, sometimes only momentary. The special committee classified dystonia based on age of onset, etiology, and distribution.
Classification by distribution includes the following categories: focal dystonia, segmental dystonia, generalized dystonia, multifocal dystonia, and hemidystonia. The term “focal dystonia” refers to involvement of a single body part. Common names, such as blepharospasm, spasmodic dysphonia, writer’s cramp, and cervical dystonia, are generally classified as focal dystonia. The term “segmental dystonia” refers to involvement of two or more contiguous areas of the body. Segmental dystonia is divided into regional categories: cranial, axial, brachial, and pedal. In this classification scheme, segmental “cranial” dystonia indicates that any combination of head and neck muscle tissue is involved. However, in reality, the neck and mandible are not part of the cranium. Therefore, for example, the combination of blepharospasm, masticatory muscle dystonia, and cervical dystonia is more accurately classified as segmental “cranio-cervical” dystonia rather than segmental “cranial” dystonia. Alternative terms like “facial-cervical” are less precise, as the masticatory muscles neither appear nor attach to the face. In fact, most facial muscles attach to the cranium and/or mandible. The term “segmental cranio-cervical” encompasses all muscle groups of that body segment, where “cranial” serves as the head-end portion of the segment and the neck forms the tail-end.
The diagnosis and anatomical classification of dystonia remain a clinical practice heavily influenced by experience and training. Regional involvement may be subtle, intermittent, and task-specific. Moreover, many muscles’ origins and insertions lie in different anatomical regions of the body (e.g., the levator scapulae, digastric, platysma, semispinalis capitis, and longissimus capitis). Therefore, in many patients, it may be challenging to clearly distinguish between “focal” dystonia and “segmental” dystonia. For example, patients with cervical dystonia often present with mild, electromyographically detectable upper thoracic paravertebral muscle involvement.
In most “blepharospasm+” subphenotype patients, using the term “segmental cranio-cervical dystonia” may be more accurate than “segmental cranial dystonia” for several reasons. First, involuntary contractions of the platysma can be observed in a significant proportion of blepharospasm patients. In these subjects, mild contractions of the platysma may be time-locked with contractions of the orbicularis oculi. The platysma is innervated by the facial nerve, which is the same nerve that innervates the orbicularis oculi muscle of the eyelid. The platysma is a broad and thin superficial muscle that extends from the upper thorax, shoulder, and clavicle up to the chin, mandible, and lower face. Second, the pharyngeal muscle tissue of blepharospasm patients may be affected, including muscles associated with jaw opening. Based on these considerations, “segmental cranio-cervical dystonia” is a more anatomically precise term that encompasses almost all previous clinical applications of the terms “Meige syndrome” and “Breughel syndrome.”

The anatomical complexity of the cranio-cervical region also hinders the use of currently available dystonia rating scales. The widely used Burke-Fahn-Marsden (BFM) rating scale includes the following regions: eyes, mouth, speech and swallowing, neck, right arm, left arm, trunk, right leg, and left leg. For each area, severity ratings range from 0 (none) to 4 (severe dystonia). Additionally, provocative factor ratings for each area range from 0 to 4. Scores for the eyes, mouth, and neck are multiplied by 0.5, while scores for speech and swallowing are multiplied by 1.0. Clearly, the BFM fails to distinctly differentiate between masticatory, lingual, and pharyngeal dysfunction. For example, in primary blepharospasm with dystonia, lingual involvement is not common. On the other hand, upper facial dystonia may occur without involving the orbicularis oculi.
Clinical Features of Segmental Cranio-Cervical Dystonia
Patients with blepharospasm may also exhibit other types of cranio-cervical involvement, including lower facial, masticatory, lingual, pharyngeal, laryngeal, or cervical dystonia. Over time, lower facial and masticatory muscle involvement in blepharospasm patients becomes quite common. Involuntary movements of the jaw and face may include lip pursing, chewing, chin jutting, grimacing, jaw opening, and closing/clenching the jaw. In some patients, movements of the jaw and chin may be rhythmic or tremor-like. Furthermore, involuntary jaw and chin movements in blepharospasm patients may not lead to sustained postures, and thus may not meet the definition of dystonia. Instead, these involuntary movements are often described as movement disorders, a poorly defined term encompassing several forms of involuntary movement (e.g., chorea, ballismus, and myoclonus), each with a more precise meaning.
Most of the time, cranio-cervical dystonia begins locally. However, over time, the dystonia may spread to other muscles of the body. Most commonly, dystonia spreads to adjacent muscles. For example, when blepharospasm does spread, it typically spreads to the lower face and/or masticatory muscles. Cranio-cervical dystonia rarely spreads to the muscles of the arms and legs. Although the probability of spreading is highest within the first three years after the onset of dystonia, spreading can occur even ten years or more later. In blepharospasm patients, older age of onset, female gender, and a history of head trauma may increase the risk of spreading. Overall, patients who initially present with eyelid dystonia (i.e., blepharospasm) have at least a 50% probability of spreading over their lifetime.
Segmental cranio-cervical dystonia should be diagnosed and treated by clinicians with extensive experience in movement disorders. Occasionally, patients with segmental cranio-cervical dystonia may be treated by general ophthalmologists and otolaryngologists without thorough investigation of secondary or neurodegenerative causes. For instance, segmental cranio-cervical dystonia may be associated with neurodegenerative diseases such as spinocerebellar ataxia or progressive supranuclear palsy. Typically, segmental cranio-cervical dystonia is caused by medications that block dopamine receptors in the brain, such as antiemetics (e.g., metoclopramide) or antipsychotics (e.g., fluphenazine). Often, brain MRI scans and blood tests are ordered to rule out the possibility of hereditary or secondary causes, such as Wilson’s disease or ischemic stroke.
Etiology and Pathophysiology of Segmental Cranio-Cervical Dystonia
The pathophysiology of Meige’s syndrome remains unclear. A series of evidence suggests that the pathophysiology of dystonia involves the striatum, with striatal activity being modulated by the dopaminergic system among other neurotransmitters. The most widely accepted hypothesis for the pathogenesis of MS is dopaminergic and cholinergic abnormalities. Recent studies using neurophysiological and neuroimaging techniques support that environmental triggers and genetic susceptibility lead to plastic changes and reduced cortical inhibition. Voxel-based morphometric analysis has investigated changes in brain structure abnormalities in MS patients and their clinical significance. This research indicates that the basal ganglia and motor cortex are involved in the pathophysiology of MS, with the precuneus participating in the development of MS. Positron emission tomography shows reduced blood flow in the sensory-motor areas due to reduced facial vibration, indicating abnormal sensory-motor processing in MS patients.
The role of the basal ganglia and thalamus is crucial for coordinating eyelid movement, the blink reflex, as well as the cerebellar-thalamic cortical pathway and the cortical-striatal-pallidal cortical pathway, as shown in functional MRI studies. The blink reflex and masseter inhibition reflex have also been studied in patients with BSP and MS. Studies have found that reflexive blinking in BSP is associated with increased activation of the caudate nucleus and sensory-motor cortex, while reduced neural responses during cerebellar reflexive blinking during the disease duration suggest a loss of inhibition within the sensory-motor cortical-basal ganglia network.
Relatively few hypothesis-driven pathophysiological studies have specifically focused on segmental cranio-cervical dystonia or included subjects with the blepharospasm plus phenotype. However, since focal dystonia shares common genetic and physiological bases, studies on blepharospasm, masticatory dystonia, and cervical dystonia are evidently related to our understanding of segmental cranio-cervical dystonia. Physiological studies indicate abnormal excitability of brainstem interneuron pathways and sensory-motor cortical areas in patients with cranio-cervical focal dystonia. The blink reflex and masseter inhibition reflex have been studied concerning brainstem interneuron pathways. The R2 component of the blink reflex and the SP2 component of the masseter inhibition reflex are enhanced in patients with cranio-cervical dystonia. Even subjects with isolated cervical dystonia exhibit enhanced R2 recovery, indicating that abnormal interneuronal excitability extends beyond the clinically involved regions. Dresel and colleagues used silent event-related functional MRI to compare three experimental groups: isolated blepharospasm, blepharospasm plus oromandibular dystonia, and a control group. During a whistling task, both dystonia groups exhibited increased activation in the sensory and tail auxiliary motor cortices. The shortening of the cortical silent period in the blepharospasm and blepharospasm plus oromandibular dystonia experimental groups is consistent with low excitability of cortical inhibitory neurons in cranial dystonia.
So far, no specific pathogenic genes for MS, BSP, and OMD have been identified; however, given the close relationship between MS, BSP, OMD, and dystonia, susceptibility genes for dystonia may play an important role in the disease’s development.
Differential Diagnosis
1. Myasthenia Gravis: However, myasthenia gravis patients may have bilateral eyelid weakness, not only difficulty in opening the eyes but also difficulty in closing the eyes, without blepharospasm, and can manually open the eyelids, with easy fatigability, symptoms that are mild in the morning and worsen in the evening, and a positive edrophonium test. The position of the eyeballs in myasthenia gravis patients may change to compensate for the fatigue of the eye muscles when blinking, while blepharospasm leads to involuntary eyelid spasms due to the fatigue of the extraocular muscles. 2. Hemifacial Spasm: Meige’s syndrome often begins with bilateral blepharospasm and gradually involves facial muscles, with eyelids often closed, difficulty in opening, and may be accompanied by involuntary contractions of the oromandibular muscles, severely affecting the whole body; while hemifacial spasm typically presents as unilateral facial muscle involuntary, irregular paroxysmal contractions, of short duration, without involuntary movements of the mouth and mandible. Even in rare cases of bilateral hemifacial spasm, the contractions of the facial muscles on both sides are asynchronous. Another distinguishing feature is that blepharospasm patients have a lowered brow (Charcot’s sign) when closing their eyes, while hemifacial spasm patients have an elevated brow with each contraction (another Babinski sign). 3. Tardive Dyskinesia: Typically caused by long-term oral antipsychotic medication, typical cases involve muscle groups that present with a stereotypical, slow, writhing pattern rather than spasms and primarily involve the oral and lingual muscles, which can aid in diagnosis based on relevant disease history and medication history. However, a few patients may also present with features of Meige’s syndrome, classified as secondary. 4. Ocular Apraxia: Common in Parkinson’s disease, progressive supranuclear palsy, etc., characterized by supranuclear involvement, where patients find it difficult to consciously open their eyes, especially when light is stimulated or when trying to gaze at something, and the difficulty in opening the eyes is more pronounced when touching the face, with contraction of the frontalis muscle but no contraction of the orbicularis oculi, and no blepharospasm. 5. Age-related Ptosis: Weakness of the levator palpebrae muscle, atrophy of eyelid fat and ligaments, head tilt when viewing, with no blepharospasm and oromandibular spasms. 6. Others: In addition to blepharospasm and oromandibular dystonia, Meige’s syndrome is also commonly associated with mood disorders, with a high incidence of depression, and some patients may also experience anxiety with depression, needing to be differentiated from primary psychiatric disorders.
Treatment of Cranio-Cervical Segmental Dystonia
Segmental cranio-cervical dystonia is typically treated with botulinum toxin injections. Successfully treating dystonia with botulinum toxin is both an art and a science. The results depend on accurately targeting the affected muscles with the appropriate amount of toxin. Electromyographic guidance is frequently used when injecting the masticatory, laryngeal, and cervical muscles. Unfortunately, many patients with segmental cranio-cervical dystonia are forced to see two or more doctors for botulinum toxin injections. For example, in the worst case, a patient may see an ophthalmologist for blepharospasm, an otolaryngologist for spasmodic dysphonia, and a neurologist for cervical dystonia. In patients with blepharospasm, attention to associated masticatory and lower facial involvement often varies significantly depending on the skill and experience of the treating physician and the expectations of the patient. Injecting botulinum toxin into the periorbital, masticatory, and platysma muscle tissues of coexisting blepharospasm patients can provide significant subjective clinical benefits with low risks of side effects.
Botulinum toxin type A has been approved by the U.S. Food and Drug Administration (FDA) for the treatment of blepharospasm and cervical dystonia, and type B botulinum toxin has been approved for the treatment of cervical dystonia. In the U.S., injections into the mandible, masticatory, laryngeal, and lingual muscle tissues are non-label applications of botulinum toxin. Compared to isolated blepharospasm, the subjective and objective improvement effects of patients with blepharospasm plus subtype after botulinum toxin injections are poorer. For example, Van den Bergh and colleagues reported significant improvement in blepharospasm patients (79%), but only moderate improvement in “Meige syndrome” patients. On average, blepharospasm plus subtype patients may experience more severe blepharospasm than those with isolated blepharospasm. Additionally, blepharospasm may be accompanied by opening or closing muscle dystonia. Compared to closing muscle dystonia, it may be more challenging to treat opening muscle dystonia with botulinum toxin injections.
A large number of oral medications have been used to treat segmental cranio-cervical dystonia; however, there are currently no multicenter, placebo-controlled, double-blind studies available for reference in this area. A single-center, double-blind crossover study indicated that sodium valproate was not beneficial for “Meige syndrome.” Similarly, a randomized, placebo-controlled single-center crossover trial indicated that spirulina was not an effective method for treating patients with blepharospasm or “Meige syndrome.” Recent publications describe benefits of zolpidem and levetiracetam in isolated cases of “Meige syndrome.” Unfortunately, commonly used medications such as anticholinergic drugs (e.g., benztropine and trihexyphenidyl), benzodiazepines (e.g., clonazepam, lorazepam), baclofen, and prochlorperazine typically yield only moderate improvements. Although side effects are often a concern, anticholinergic drugs have been shown to be valuable in segmental cranio-cervical dystonia. In many patients, low-dose clonazepam is also effective. Additionally, the side effects associated with benzodiazepines are generally easier to tolerate than those of anticholinergic drugs.
In recent years, deep brain stimulation (DBS) has gained increasing attention as a treatment option for patients with refractory dystonia. Clearly, DBS is an important treatment option for DYT1 generalized dystonia patients. Currently available data suggest that DBS is also an effective treatment method for many but not all patients with segmental cranio-cervical dystonia. Most commonly, the internal segment of the globus pallidus (GPi) has become the target for primary adult-onset segmental cranio-cervical dystonia patients. Bilateral stimulation of the GPi may be required to control axial symptoms. Unlike in Parkinson’s disease and essential tremor, the benefits of DBS for dystonia typically manifest gradually over several months. Interestingly, long-term GPi DBS may correct the neural network abnormalities that lead to the onset of dystonia, suggesting that continuous stimulation may ultimately prove unnecessary.
DBS is associated with the development of stroke, infection, and new neurological signs, including but not limited to hemiparesis, visual field defects, dysarthria, and dysphagia. In some dystonia patients, stimulation of the GPi may lead to mild deterioration of motor function in previously non-dystonic body areas. Based on these considerations, DBS should only be seriously considered for patients with segmental cranio-cervical dystonia when other treatment options have failed, under the guidance of a skilled and experienced neurologist.
References:
https://www.chunyuyisheng.com/pc/topic/210641/
https://www.frontiersin.org/journals/neurology/articles/10.3389/fneur.2021.630221/full
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2743078/
https://www.sohu.com/a/393118592_120051481
https://drthindhomeopathy.com/disease/meige-syndrome/
https://www.myupchar.com/en/disease/meige-syndrome
https://medlexi.de/Meige-Syndrom
The management of general pain and sensory abnormalities has been deeply studied by our team under the guidance of Professor Wu Xirui of Hebei Medical University, through years of clinical exploration, integrating traditional treatment methods with modern basic research, forming a comprehensive treatment and theoretical system. For different symptoms, disease courses, and clinical characteristics, we have successively developed “one-needle analgesia” technology, “intradermal/subcutaneous acupuncture” technology, “trigger point therapy,” and “fascia stretching techniques, which have broad indications, quick effects, less pain, and low costs, and have been widely praised by doctors and patients.
Expert Introduction Real Cases
Real Cases