
Bletilla striata polysaccharide (BSP) is a polysaccharide extracted from the medicinal material Bletilla striata, composed of mannose and glucose (2.4:1) linked by β-glycosidic bonds. It exhibits biological activities such as anti-inflammation, antioxidant, and anti-fibrosis [1], and is non-toxic, biodegradable, and highly compatible, making it widely used in drug formulation [2-6]. Additionally, literature reports that BSP can be effectively recognized by the mannose receptors and β-glucan receptors on the surface of macrophages, thus, preparing BSP carriers can be used for macrophage targeting [7-8]. However, as a drug carrier, BSP has strong water solubility, which limits the encapsulation of hydrophobic drugs. Many researchers have attempted to modify its hydrophobicity using substances such as stearic acid, cholesteryl succinate, and sulfates [9-13].
This experiment modifies BSP with reactive oxygen species (ROS) responsive group 4-(hydroxymethyl) phenylboronic acid pinacol ester (PBAP) [14] to obtain ROS-sensitive BSP (Oxi-BSP). This new material has an amphiphilic structure, with PBAP as the hydrophobic segment and BSP as the hydrophilic segment. This material can spontaneously form micelles in aqueous solution [18]; and using the antioxidant curcumin (Cur) as a model drug for encapsulation, we prepared ROS-sensitive micelles loaded with Cur, where BSP can specifically recognize the receptors on the surface of Kuffercell (KC). After specific uptake by KC, under high concentrations of ROS in KC, the phenylboronate is oxidized, the chemical bonds break, and the drug is released to exert its antioxidant effect.
1 Instruments and Reagents
Lambda 35 UV-visible spectrophotometer, Perkin Elmer; 78HW-1 digital constant temperature magnetic stirrer, Hangzhou Instrument Motor Co., Ltd.; Master-sizer 2000 laser particle size analyzer, Malvern, UK; BT125D electronic balance, Sartorius, Germany; Solebag dialysis bag MD34, with a cut-off molecular weight of 8000-14000; BSP, mass fraction 90%, Xi’an Langze Biotechnology Co., Ltd.; PBAP, N,N‘-carbonyldiimidazole (CDI), 4-dimethylaminopyridine (DMAP), McLin Reagent Co.; Cur raw material, mass fraction 98%, batch number 201406, Shanghai Aladdin Biochemical Technology Co., Ltd.; DMSO, Shanghai Aladdin Biochemical Technology Co., Ltd.; other reagents are analytically pure, and water is deionized water produced in the laboratory; Cur reference substance, mass fraction 98.9%, batch number 110823-201405, China Food and Drug Administration Institute.
2 Methods and Results
2.1 Activation of PBAP
0.500 g of PBAP was dissolved in 15 mL of anhydrous dichloromethane, then 0.692 g of CDI was added, and the reaction was stirred for 30 minutes. The mixture was diluted with dichloromethane and washed three times with 10 mL of double-distilled water, discarding the aqueous layer. The organic phase was washed with 12 mL of saturated sodium chloride solution and the sodium chloride layer was discarded. It was dried over anhydrous sodium sulfate, and after vacuum drying and concentration, a pure white solid was obtained, which was the activated 4-(hydroxymethyl) phenylboronic acid pinacol ester (CDI-PBAP), as shown in Reaction Scheme 1.
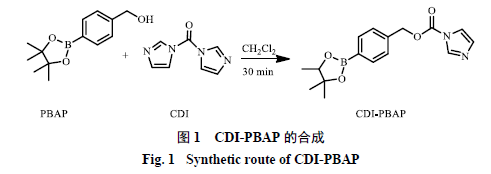
2.2 Modification of Bletilla Polysaccharide
762.8 mg of BSP was placed in a 50 mL round-bottom flask, dissolved in 8 mL of anhydrous DMSO, then 0.466 g of DMAP was added, followed by 1.000 g of CDI-PBAP. The mixture was stirred overnight. The modified BSP was precipitated in 10 mL of double-distilled water, and then separated by centrifugation at 10,200 × g for 15 minutes. The separated product was washed twice with 10 mL of double-distilled water, and then the supernatant was removed by centrifugation. Freeze-drying was performed to remove residual moisture, yielding Oxi-BSP, as shown in Reaction Scheme 2.
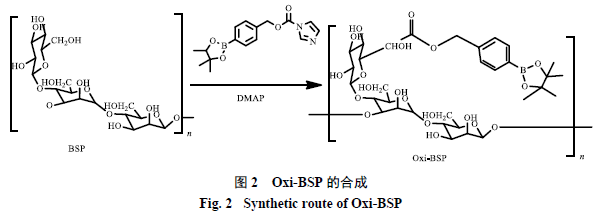
2.3 Structural Characterization
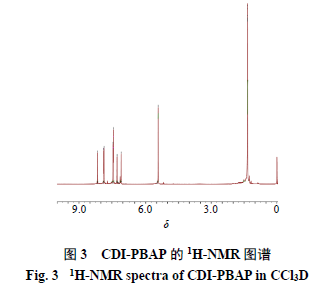
2.3.1 Characterization of CDI-PBAP The 1H-NMR spectrum was measured using a Bruker Avance 400 MHz NMR spectrometer (Switzerland Bruker Company), results shown in Figure 3. The infrared spectra (IR) of reactants and polymers were measured using a micro-infrared spectrometer (VERTEX70), with a wavenumber range of 4000-400 cm−1, results shown in Figure 4. 1H-NMR (400 MHz, CDCl3) δ: 8.15 (1H, s), 7.86 (2H, d, J = 7.6 Hz), 7.44 (2H, d, J = 7.6 Hz), 7.27 (1H, s), 7.07 (1H, s), 5.43 (2H, s), 1.35 (12H, s). In the IR spectrum, the peak around 3300 cm−1 disappears from PBAP to CDI-PBAP, indicating that -OH in the raw material has reacted. At 1750 cm−1, the carbonyl peak appears, and the characteristic peaks of the benzene ring in the range of 1500-1000 cm−1 indicate that -OH on PBAP has connected with -COOH on CDI. The above results indicate that the structure of the synthetic product is consistent with the target compound.
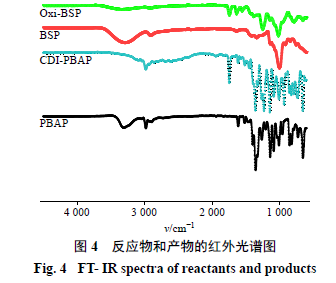
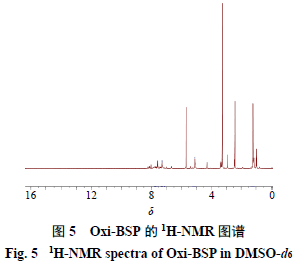
2.3.2 Characterization of Oxi-BSP The method is the same as in section “2.3.1”, results shown in Figure 5. 1H NMR (400 MHz, CDCl3) δ: 7.59 (2H, d, J = 7.6 Hz), 7.29 (2H, d, J = 7.6 Hz), 5.10 (2H, s), 1.21 (12H, s) are the 4Hs on the benzene ring, 2Hs on the -CH2 of hydroxymethyl, and 12Hs on the -CH3. In the IR spectrum, the appearance of the ester bond group around 1730 cm−1 indicates that PBAP has been connected to BSP, and many peaks of PBAP appear, such as the appearance of benzene ring characteristic peaks, proving that the product is the target compound.
2.4 Preparation of Oxi-BSP Drug-Loaded Micelles
Accurately weigh 50 mg of Oxi-BSP and 5 mg of Cur, dissolve in 5 mL of organic solvent (methanol-DMSO 1:1), and stir magnetically to mix thoroughly. Transfer the mixed solution into a dialysis bag with a cut-off molecular weight of 8000-14000, and place it in 500 mL of deionized water for dialysis, replacing with fresh deionized water at 2, 4, 6, 8, 12, and 24 hours. After dialysis, the solution in the dialysis bag is centrifuged at 3000 r/min for 10 minutes, and the supernatant is filtered through a 0.45 μm microporous filter to obtain the drug-loaded micelle aqueous solution.
2.5 Determination of Drug Loading and Encapsulation Rate[19-20]
Accurately pipette 1 mL of micelle solution into a 100 mL volumetric flask, add methanol to the mark, then ultrasonicate for 5 minutes to disrupt the micelle structure. Filter through a 0.45 μm microporous filter and measure the A value, calculate the drug mass concentration according to the regression equation obtained from the standard curve, and calculate the encapsulation rate and drug loading using the formulas.
Encapsulation rate = (Wamount of drug in micelles/Wamount of drug added)
Drug loading = (Wamount of drug in micelles/Wmicelle mass)
2.5.1 Determination of Detection Wavelength Take an appropriate amount of Cur reference substance and blank micelles, dissolve in anhydrous ethanol and dilute to an appropriate concentration, then filter. Perform UV scanning at a wavelength of 200-800 nm. The Cur reference substance has a maximum absorption wavelength at 422 nm, and the blank micelles have no absorption, thus 422 nm is determined as the detection wavelength for Cur.
2.5.2 Plotting of Standard Curve Take an appropriate amount of Cur reference substance, prepare a series of reference solutions with different mass concentrations using anhydrous ethanol, and measure the A values. Perform linear regression of the A values (Y) against their mass concentrations (X) to obtain the regression equation Y = 0.1436 X – 0.0116, r2 = 0.9999, indicating a good linear relationship between Cur and A values in the range of 1.194-7.164 μg/mL.
2.5.3 Precision Test Take three groups of different mass concentrations of Cur reference substance solution (low, medium, high), and measure the A values every 2 hours within 1 day to calculate intraday precision. Measure continuously for 6 days to calculate interday precision. The intraday precision RSD values for low, medium, and high groups are 1.02%, 1.01%, and 0.894%, respectively, and the interday precision values are 1.94%, 0.538%, and 0.541%, indicating good precision of this method.
2.5.4 Recovery Rate Test Take 1 mL of blank micelles, add 0.8, 1.0, and 1.2 mL of Cur solution with a mass concentration of 1.426 mg/mL, place in a 50 mL volumetric flask, and dilute with methanol to the mark. Take 1 mL into a 10 mL volumetric flask, dilute to the mark, and measure the A value. The recovery rate is calculated to be 98.97%, with an RSD of 2.1%, indicating high accuracy of this method.
2.6 Single Factor Investigation
2.6.1 Determining Dialysis Temperature Under fixed conditions, place the drug and carrier solutions at 4, 12, 20, 28, and 37 °C, stir and dialyze for 24 hours to prepare drug-loaded micelles, using the same preparation method as in section “2.4”, and measure their particle size and drug loading using the method in section “2.5”. The results show particle sizes of 117.60, 96.52, 77.16, 134.20, and 121.00 nm, and drug loading of 8.510%, 7.231%, 8.620%, 7.483%, and 6.031%, respectively.
2.6.2 Selecting Dialysis Time Samples were taken from the dialysis bag at 12, 18, 24, 30, and 36 hours to prepare drug-loaded micelles, using the same preparation method as in section “2.4”, and measuring their particle size and drug loading using the same method. The results show particle sizes of 80.91, 114.20, 77.16, 133.30, and 190.80 nm, and drug loading of 8.790%, 8.426%, 8.620%, 7.615%, and 4.348%, respectively.
2.6.3 Selecting Dosage Keeping the amount of carrier and other conditions fixed, prepare drug-loaded micelles with different dosages of 5, 7.5, 10, 12.5, 15, 17.5, and 20 mg, using the same method as in section “2.4”, and measure their particle size and drug loading using the method in section “2.5”. The results show particle sizes of 77.16, 155.10, 163.00, 214.70, 215.60, 222.50, and 232.70 nm, and drug loading of 8.620%, 11.090%, 10.670%, 14.640%, 16.380%, 12.170%, and 7.100%, respectively.
2.6.4 Selecting Dialysis Medium Keeping other conditions fixed, prepare drug-loaded micelles using deionized water, saline, and PBS solution at pH 5.0, using the same method as in section “2.4”, and measure their particle size and drug loading using the method in section “2.5”. The results show particle sizes of 77.16, 0 (indicating no micelles formed), and 0 nm, and drug loading of 91.920%, 0, and 0, respectively.
2.6.5 Screening the Mixing Ratio of Organic Solvents (DMSO and Methanol) Keeping other conditions fixed, prepare drug-loaded micelles with DMSO and methanol ratios of 1:1, 1:4, and 4:1, using the same method as in section “2.4”, and measure their particle size and drug loading using the method in section “2.5”. The results show particle sizes of 77.16, 159.10, and 0 nm, and drug loading of 91.920%, 69.430%, and 0, respectively.
2.7 Orthogonal Design Method to Optimize Formulation and Preparation Process
2.7.1 Orthogonal Design Based on the results of single-factor investigation, select three main parameters affecting the drug loading of Oxi-BSP drug-loaded micelles for investigation, namely dialysis time (A), dialysis temperature (B), and dosage (C), setting 3 levels for each factor. According to the orthogonal design L9(33) table, experiments were conducted to optimize the best formulation and preparation process, with factor levels shown in Table 1.
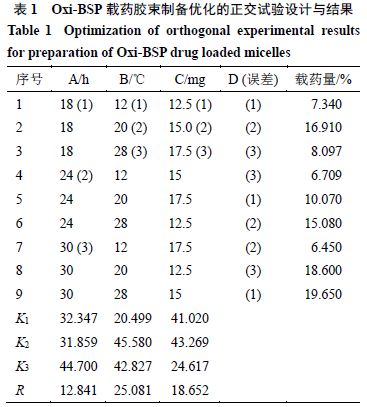
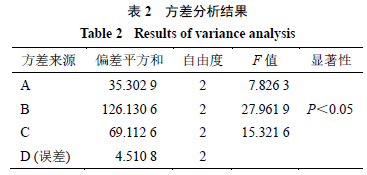
2.7.2 Result Analysis Based on the conditions in Table 1, prepare Oxi-BSP drug-loaded micelles and determine the drug loading, results shown in Table 1, with variance analysis results shown in Table 2.
From Table 1, it can be seen that the influence of various factors on drug loading is in the order of B > C > A, indicating that the dialysis temperature has the greatest influence on drug loading, followed by dosage, with the least influence from dialysis time.
From Table 2, it can be seen that the influence of dialysis temperature on drug loading has significant differences (P < 0.05), while there is no significant difference in dialysis time at the selected levels. Considering aspects such as economic efficiency, the dialysis time can be adjusted to 18 hours; therefore, the optimal process conditions are A1B2C2, with a dialysis time of 18 hours, a dosage of 15 mg, and a dialysis temperature of 20 °C.
2.8 Verification Experiment
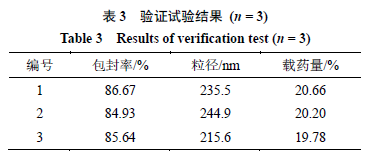
Combining the established process and parameters, prepare 3 batches of Oxi-BSP drug-loaded micelles according to the best formulation and preparation process conditions, measure the particle size, encapsulation rate, and drug loading, results shown in Table 3. From the table, it can be seen that the average drug loading under optimized process conditions is (20.21 ± 0.44)%, and is relatively stable, with encapsulation rate and particle size being ideal, indicating that this method is feasible and the process has good reproducibility.
2.9 Characterization of the Physical and Chemical Properties of Oxi-BSP Drug-Loaded Micelles
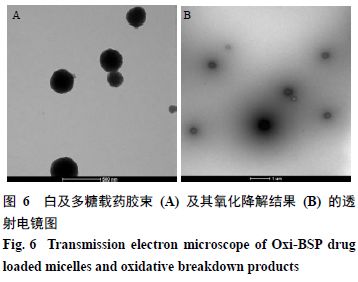
2.9.1 Morphological Study Under the optimal formulation and preparation process conditions, drug-loaded micelles were prepared, diluted to a certain multiple with deionized water, dropped onto copper grids covered with carbon film, dried, and observed under a transmission electron microscope. The results are shown in Figure 6-A, indicating that Oxi-BSP drug-loaded micelles are spherical, uniformly sized, and well-formed.
2.9.2 Oxidative Degradation of Drug-Loaded Micelles Take an appropriate amount of drug-loaded micelles, add hydrogen peroxide to simulate the in vivo environment, and incubate for 6 hours, results shown in Figure 6-B. It can be seen that the boundary of the micelles becomes blurred, indicating that the micelle structure has been damaged, while the particle size distribution PDI is 1.000, indicating that the micelles have been destroyed and the particle size is no longer uniform, preliminarily judging that the Oxi-BSP carrier material has oxidative responsiveness.
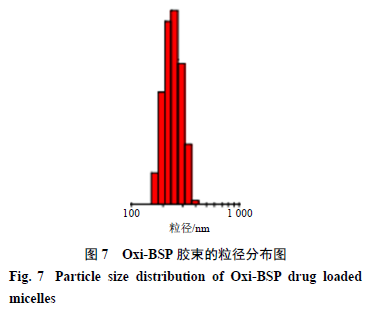
2.9.3 Particle Size and Size Distribution Take the Oxi-BSP drug-loaded micelles, dilute with deionized water, and measure their particle size and size distribution using a laser particle size analyzer, results shown in Figure 7, with an average particle size of 225.33 nm and a polydispersity index (PDI) value of 0.081.
3 Discussion
This experiment uses biodegradable BSP as the raw material, and through the use of ROS responsive group PBAP to modify the -OH, synthesized a new material that has both hydrophilic and hydrophobic groups. This material can spontaneously form micelles in aqueous solution, and using Cur as a model drug for encapsulation, successfully prepared drug-loaded micelles.
Regarding the choice of preparation method, this experiment investigated film hydration method [21], solvent evaporation method [22], and dialysis method [23]. Since the organic solvents used were DMSO and methanol, and DMSO has a high boiling point and is difficult to evaporate, film hydration and solvent evaporation methods could not completely remove DMSO, but DMSO can mix with water, hence the dialysis method was used to remove the organic solvent and prepare drug-loaded micelles.
From the results of single factors, it can be seen that as the dialysis time increases, the drug loading decreases, possibly due to the instability of the micelle structure over time. Considering that DMSO had not been completely dialyzed out after 12 hours, the dialysis time was controlled to around 18 hours; at 4 °C, DMSO was difficult to diffuse out of the dialysis bag, and some Cur dissolved in DMSO, resulting in higher measured drug loading and deviation in results. When the temperature exceeds 20 °C, drug loading significantly decreases; therefore, the dialysis temperature was controlled around 20 °C. Dosage has a significant impact on drug loading, and when the dosage exceeds 15 mg, drug loading significantly decreases, thus the dosage was controlled around 15 mg. When the dialysis medium is PBS or saline, micelles cannot be formed, possibly due to the macromolecular material of the carrier exhibiting a salt-out effect, further investigation is needed.
Based on the single factor investigation, an orthogonal test design was conducted to select the factors that significantly affect the drug loading of Oxi-BSP drug-loaded micelles, optimizing the best formulation and preparation process to a dosage of 15 mg, dialysis time of 18 hours, and dialysis temperature of 20 °C. Verification found that this process is superior, reasonable, and highly reproducible, serving as a practical and effective means for screening formulations and processes.
References (Omitted)
Source: Tingting Dang, Ji Wang, Peng Tang, Xiaojuan Wang. Preparation and characterization of ROS-responsive Bletilla striata polysaccharide drug-loaded micelles [J]. Chinese Herbal Medicine, 2018, 49(23):5548-5553.

