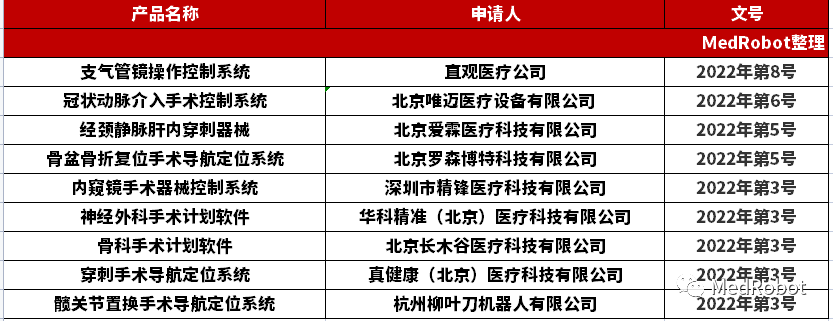
MedRobot today reviews the surgical robots related medical devices that have submitted materials and entered the NMPA innovation channel in 2022. The scope is document number 2022 No. 1-9 (No. 9 was announced in January 2023). Since the public documents only include the applicant and product name (the red part below), and the products are not on the market, the content we queried may not be accurate, and is for reference only.
Overall, there are 9 models, including the surgical robots themselves and related software and consumables, categorized as follows:
01
Surgical Robot
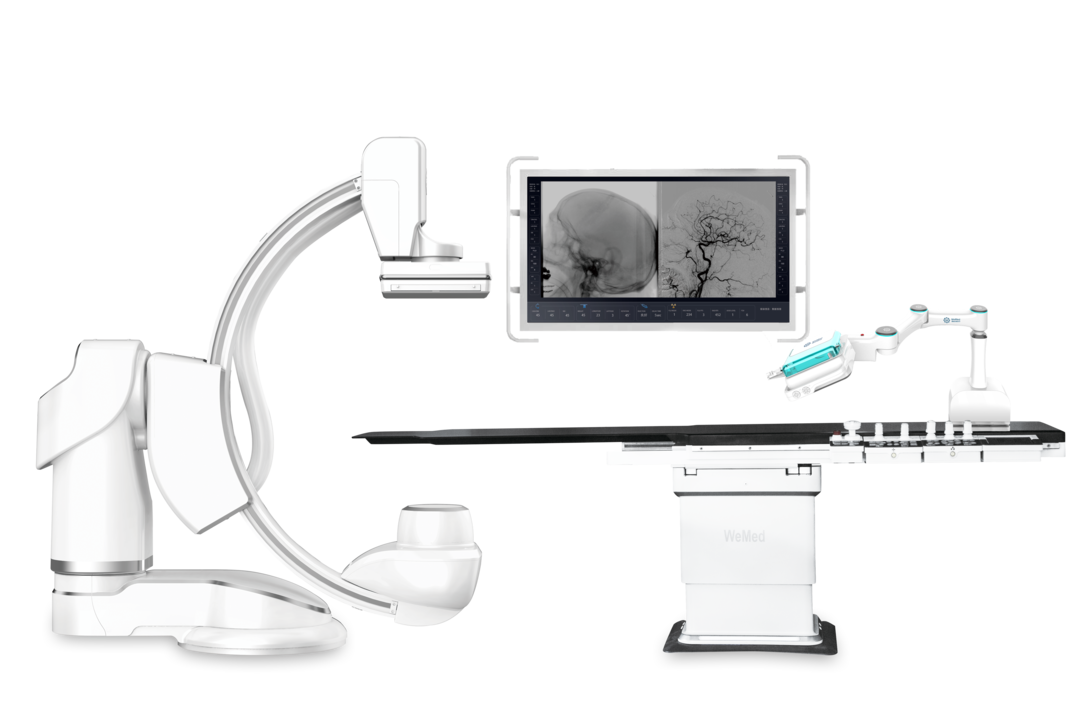
Product Name: Coronary Intervention Surgery Control System
Applicant: Beijing Weimai Medical Equipment Co., Ltd.
Weimai Medical was established in 2014, focusing on interventional diagnostic and therapeutic equipment and full-process solutions, with years of experience in digital vascular imaging product R&D design and industrialization, covering multiple interventional product lines such as large vascular angiography machines (DSA), mobile X-ray C-arms, integrated mobile X-ray C-arms, and interventional robots.
Weimai Medical’s ETcath vascular interventional surgical robot reportedly completed the first percutaneous coronary intervention surgery (PCI) at Anzhen Hospital by Professor Zhou Yujie’s team in October 2021. In 2022, Weimai Medical will closely integrate with clinical practices to explore and develop the interactive application scenarios of DSA + interventional robots, and innovate AI technologies for intelligent imaging and treatment solutions, thereby creating a collaborative system of “eye, hand, and brain” for interventional diagnosis and treatment.
Product Name: Pelvic Fracture Reduction Surgery Navigation System
Applicant: Beijing Rosenbot Technology Co., Ltd.
Rosenbot was established in 2017, as a product of the close integration of Beijing University of Aeronautics and Astronautics and Beijing Jishuitan Hospital, focusing on original innovations in cutting-edge medical robotics technology, empowering medical institutions with original medical robot technology. The company is committed to building a new generation of intelligent orthopedic surgical robot systems with fracture reduction capabilities, achieving real-time 3D navigation during surgery, assisting in fracture reduction operations, and automatic surgical planning, thus realizing intelligent surgical operations from closed reduction of fractures to minimally invasive fixation, meeting the urgent clinical needs.
International experts unanimously agree that achieving a fracture displacement of less than 10mm through reduction for pelvic fractures is excellent; through the Rosenbot surgical robot, the maximum post-operative displacement of the fracture end is only 3.41mm, significantly better than the accuracy of manual reduction, achieving “precision”, “minimally invasive”, and “intelligent”. Since closed reduction does not require a large incision, the surgical time is shorter, the trauma is smaller, and the internal fixation consumables used are also cheaper, significantly shortening the patient’s hospital stay and postoperative recovery time, reducing medical costs.
Rosenbot completed over 100 million RMB in Series B financing, aiding the intelligent development of surgical robots
2022 Annual Review of Surgical Robots: Rosenbot
Product Name: Hip Replacement Surgery Navigation System
Applicant: Hangzhou Lancet Robot Co., Ltd.
Lancet Robot was established in 2018, focusing on hip replacement, knee replacement, oral implantation, and vascular intervention across four surgical tracks. At the same time, it can provide hospitals with AI-assisted diagnosis, personalized surgical planning, surgical navigation robot systems, postoperative evaluation, and other full-process solutions.
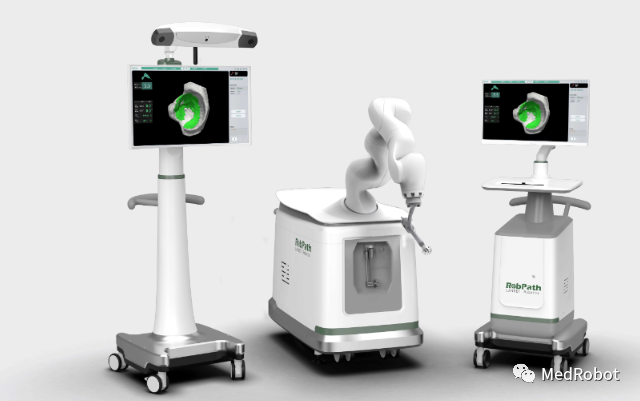
The LancetRobPathTHA open surgical platform achieves efficient point cloud registration, fast and precise automatic positioning, real-time display of grinding status, three-dimensional boundary control, and sub-millimeter level precision for safe and efficient grinding, with a power-off protection mode to avoid grinding beyond the planned range. It improves the intelligent level of surgery, data accumulation, and analysis capabilities, integrating patients’ anatomical and physiological characteristics, prosthetic design, and surgical techniques, utilizing digital advantages to achieve personalized prosthetic selection, surgical schemes, and operations.
True Health was established in 2018, focusing on medical robots as core products, concentrating on precise diagnosis and minimally invasive treatment of tumors, assisting medical institutions and doctors at all levels. The True Health puncture surgical robot has core functions such as intelligent lesion identification, puncture path planning, surgical environment perception, robotic arm-assisted puncture, and respiratory tracking system, assisting doctors in achieving safe and precise lesion puncture, providing patients with minimally invasive diagnostic and therapeutic services, comprehensively solving the challenges of soft tissue percutaneous puncture.
Under the guidance of the puncture surgical robot, the microwave ablation needle accurately reaches the target position for lung tumor treatment.
In May 2022, the “Puncture Surgery Navigation System” Class III medical device registration certificate was approved.
Product Name: Bronchoscope Operation Control System
Applicant: Intuitive Surgical (Shanghai) Co., Ltd.
The Bronchoscope Operation Control System Ion, independently developed by Intuitive Surgical, is another new original product following the da Vinci surgical robot. Ion has entered the innovation channel, expected to accelerate its listing process in China. Ion uses bronchoscopy to assist in guiding catheters and endoscopic tools in the pulmonary respiratory tract, with a catheter inner diameter of 2mm compatible with various biopsy tools for diagnosis and treatment. The visual probe can be inserted into the catheter, achieving real-time display during navigation, and the robotic arm-controlled catheter head can achieve precise positioning during biopsy, with built-in shape sensors in the catheter body providing instant feedback on shape and position during navigation and biopsy.
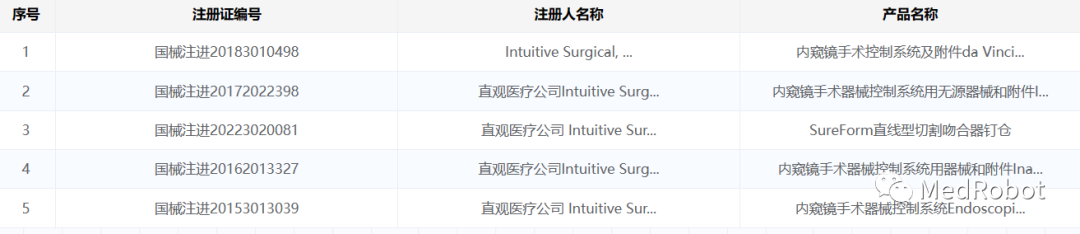
MedRobot adds that Intuitive Surgical has been approved for registration in China as shown in the table below. Its single-port robot SP has not entered China and has not entered the innovation channel. Ion is the first product reported in the innovation channel.
02
Related Instruments
Product Name: Neurosurgery Surgical Planning Software
Applicant: Huake Precision (Beijing) Medical Technology Co., Ltd.
Huake Precision was established in 2015, and the team has developed more than ten products including various medical robots, medical navigation, medical software, medical electrodes, and medical lasers. The company team began successful attempts at intelligent medical innovation in 2003, gradually achieving over 90% localization in this field through independent technological innovation and industrialization of intracranial electrodes, supporting over 5,000 related surgeries annually, directly promoting the development of functional neurosurgery in China.
This product is primarily used in the field of neurosurgery and is China’s first highly compatible multimodal imaging fusion neurosurgery surgical planning independent software, achieving international leading levels in key technologies such as multi-modal imaging data processing, segmentation fusion, registration, and brain drift correction, solving the compatibility issues between existing domestic surgical planning software and Leksell head frames, navigation, and surgical robots, providing neurosurgeons with a powerful, efficient, and intelligent reliable assistant, especially for minimally invasive localization and precise resection of intracranial small lesions, deep lesions, multiple lesions, and lesions located in important functional areas, which has significant clinical value.
Neurosurgery Navigation Surgical Robot Review Report | Huake Precision
Huake Precision’s neurosurgery surgical planning software has been approved to enter the special review process for national innovative medical devices
Completed 300 million RMB financing! Neurosurgery surgical robot company completed Series D
Product Name: Orthopedic Surgery Planning Software
Applicant: Beijing Changmu Valley Medical Technology Co., Ltd.
Beijing Changmu Valley Medical Technology Co., Ltd. is a national high-tech enterprise focusing on orthopedic AI and surgical robot solutions, providing full-process solutions for hospitals in orthopedic diagnosis, personalized surgical planning, surgical robots, and postoperative evaluation. Changmu Valley® has completed six rounds of financing (Series B), with investors including domestic and foreign venture capital, such as China International Capital Corporation, IDG Capital, Dinghui VGC, Yuanhe Origin, Yuansheng Venture Capital, SoftBank China, Lenovo Capital, Zhongguancun Development Group, Lenovo Star, and Fengrui Capital.
Changmu Valley® completed 540 million RMB Series B financing
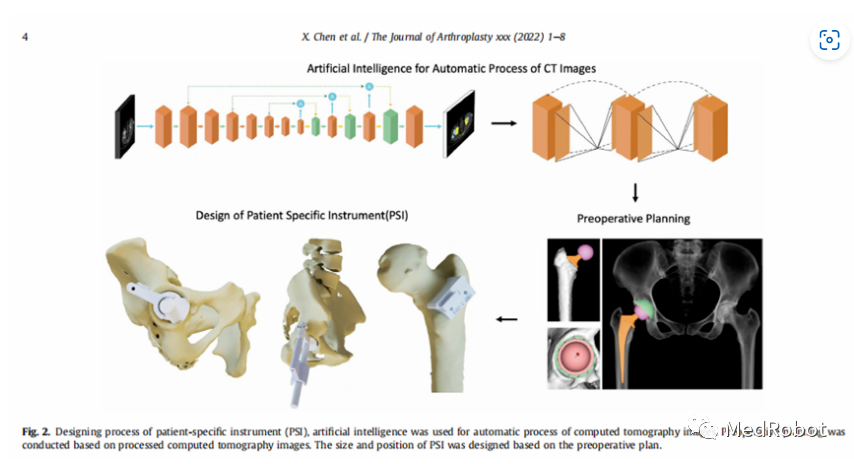
AIJOINT digitizes joint replacement, assisting in three-dimensional surgical solutions for joint replacement, providing orthopedic joint surgeons with a full-process joint replacement solution covering preoperative planning, intraoperative guidance, and postoperative follow-up.According to the company’s official website: “AIJOINT-iPSI® artificial intelligence THA group, 30 patients underwent AIJOINT® artificial intelligence three-dimensional medical imaging reconstruction based on CT, and the design of the iPSI® intelligent guide plate is based on individual patient conditions, using artificial intelligence to automatically process CT images, accurately determining the size and position of iPSI® before surgery. In the traditional THA group, 30 patients underwent AIJOINT® artificial intelligence three-dimensional medical imaging reconstruction based on CT and received Free-hand (traditional) THA surgery.”
“Compared to the ‘freehand technique’, the intelligent iPSI® assisted THA improved the accuracy of acetabular prosthesis placement. In AIJOINT-iPSI® assisted THA, there were 27 cases with anteversion angle error within 5 degrees and 25 cases with abduction angle error within 5 degrees, all cases achieved target anteversion and abduction angles within 10 degrees.”
The company’s other products include:

Product Name: Endoscopic Surgical Instrument Control System
Applicant: Shenzhen Jingfeng Medical Technology Co., Ltd.
Jingfeng Medical® is a service provider that independently develops and provides innovative solutions for surgical procedures, focusing on the design, development, manufacturing, and commercialization of surgical robots, including multi-port laparoscopic surgical robots, single-port laparoscopic surgical robots, natural orifice surgical robots, and high-end minimally invasive surgical instruments.
According to Jingfeng Medical® reports, the company has fully mastered several core technologies involved in surgical robots independently and has formed a complete intellectual property system, having applied for and authorized 438 domestic and international patents, making it the first company in China and the second globally to complete critical clinical trials for multi-port and single-port surgical robots. Jingfeng Medical’s laparoscopic surgical robot has been approved. For detailed information on products under development, please refer to the text below.
Jingfeng is once again sprinting for an IPO! Sales have been achieved!
Product Name: Transjugular Intrahepatic Puncture Instrument
Applicant: Beijing Ailin Medical Technology Co., Ltd.
Ailin Medical was established in 2018, and no financing records and website were found. Its controlling shareholder is Haijieya Medical Devices Co., Ltd. Haijieya focuses on two minimally invasive fields: “Percutaneous Intervention” and “Endovascular Intervention”, with products including the “Kangbo Knife®” tumor minimally invasive treatment system.
Since no product introduction was found on the company website, an excerpt from a related patent of the company is provided; this patent may not relate to the above product and is for reference only.
This utility model provides a puncture instrument with good anti-bending performance, preventing the doctor from causing angular bending when bending the tube, thereby ensuring the smoothness of the pipeline. The puncture instrument includes a core needle, a core needle catheter, and a sleeve. The core needle is inserted into the core needle catheter to form a first plug-in part, and the first plug-in part is inserted into the sleeve to form a second plug-in part, with the sleeve having a curved section at the distal end when in use, and the curved section being externally fitted with an anti-bending auxiliary tube to prevent the operator from causing angular bending when bending the tube.

Overview of Surgical Robots (Continuously Updated)
Overseas:
Major Companies: Medtronic | Johnson & Johnson | Smith & Nephew | Intuitive Surgical | J&J | Zimmer Biomet |
Laparoscopic: Versius | Senhance | Mira|Dexter | hinotori | Symani Mantra | ReVo-i | Curexo Orthopedic:ExcelsiusGPS | Remi | Natural Orifice:Anovo | Flex | Vascular Intervention:R-one| Puncture:Galaxy | NDR-ANT | Dental:Yomi Ophthalmology:ORYOM | Neurosurgery:LOOP-X | Others:PROCEPT | CyberKnife | HistoSonics | Theraclion |
China:
Minimally Invasive Robot | Tianzhihang | Kangnuo | Shurui | Rosenbot | Aibo Medical | Jingfeng Medical