Antibody-drug conjugates (ADCs) are formed by linking monoclonal antibodies (mAbs) with small molecule drugs through linkers. The specificity of mAbs for tumor cells allows small molecule drugs to act effectively within tumor tissues, significantly reducing the high toxic side effects of traditional small molecule drugs and enhancing overall therapeutic efficiency. Currently, there are 13 ADC drugs approved globally, and companies are increasing their investments in this area. According to Grandview data, as ADC drugs continue to be launched and the indications expand to more disease areas, the industry scale of ADC drugs is expected to maintain rapid growth, with the market size projected to reach $21 billion by 2025, and a compound annual growth rate (CAGR) exceeding 50% from 2020 to 2025.
Structure and Mechanism of ADCs
ADCs consist of three components: mAbs that can target cancer cells, highly bioactive small molecule drugs, and linkers that connect the mAbs and small molecule drugs (Figure 1). After the antibody binds to the tumor cell surface antigens, ADCs mediate their entry into the cells through endocytosis, and they are transported into lysosomes. In the lysosomes, the linker or antibody portion of the ADC is degraded, releasing the small molecule drug, which then exerts its cytotoxic effect to kill tumor cells (Figure 2).
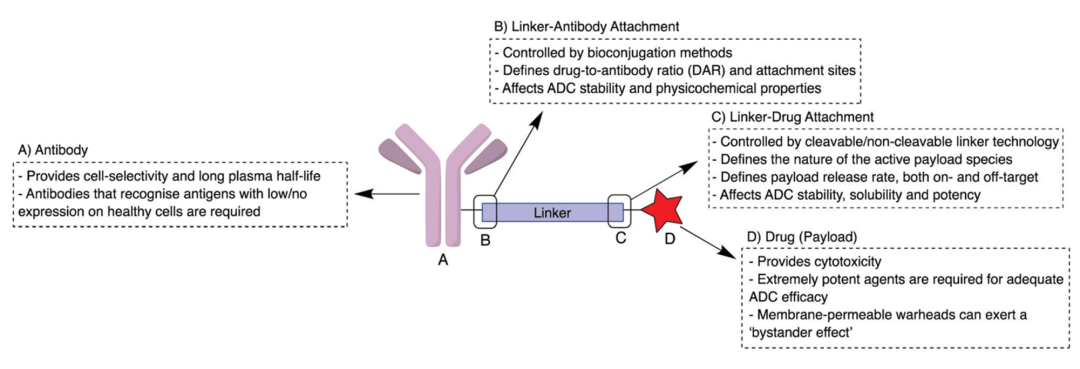 Figure 1: Structure of ADCs
Figure 1: Structure of ADCs
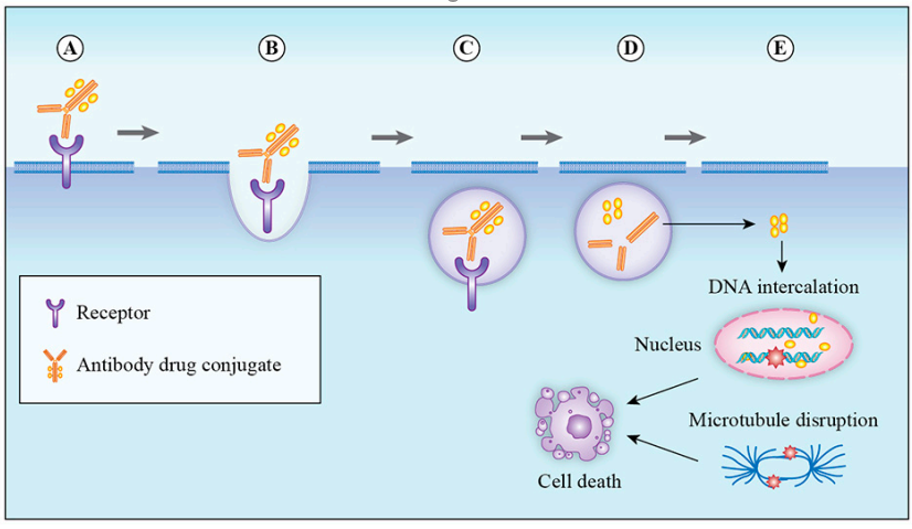
Figure 2: Mechanism of ADCs
Design Requirements for ADCs
In the early 20th century, Nobel laureate Paul Ehrlich first proposed the theory of the “magic bullet”. Since then, ADCs have made significant progress through years of research. Researchers have found that to achieve higher therapeutic efficacy, the selection of mAbs, small molecule drugs, and linkers in ADCs is crucial, and further requirements have been proposed for these three components when designing ADCs.
Antigen Selection:
To achieve lower non-specific toxic side effects and a better therapeutic index (TI), the antigen targeted by the antibody in ADCs must be highly expressed in tumor tissues and low or not expressed in normal tissues. Typically, these antigens are divided into two categories: tumor-specific antigens (TSA) that are expressed only in tumor tissues, and tumor-associated antigens (TAA) that are highly expressed in tumor tissues but low in normal tissues. Additionally, these antigens need to be expressed on the tumor cell membrane and mediate the entry of ADC drugs into the cells, allowing the small molecule drugs to exert their effects. Commonly targeted antigens include CD25, CD33, and CD174 (Figure 3).
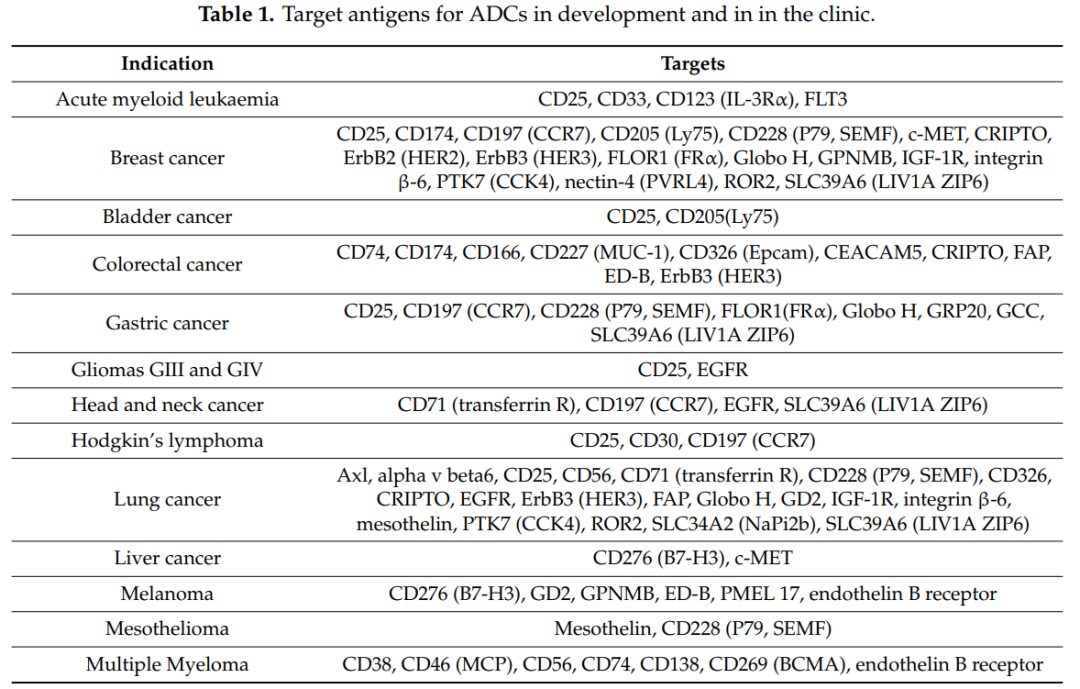
Figure 3: Commonly Used Target Antigens in Research and Clinical Practice
Antibody Selection:
Antibodies must meet high specificity, strong target binding ability, low immunogenicity, and low cross-reactivity to achieve more efficient uptake of ADCs by tumor cells and a longer serum half-life of ADCs. Currently, ADCs in clinical and preclinical studies often choose IgG as the antibody targeting the intended antigen. Among them, IgG1 is most commonly studied and used as it balances a long blood half-life and strong immune activation well and has a high natural abundance. In addition to IgG1, IgG4 is often used in ADC design that requires high immunogenic response due to its lower immune activation effects. Both IgG1 and IgG4 have 12 intrachain disulfide bonds and 4 interchain disulfide bonds, with the interchain disulfide bonds being highly reactive and often used as linker reaction sites. It is worth mentioning that IgG4 has a high Fab segment exchange property, which can lead to off-target effects, so in clinical applications of ADCs using IgG4 antibodies, the S228P variant strategy is adopted to reduce Fab segment exchange.
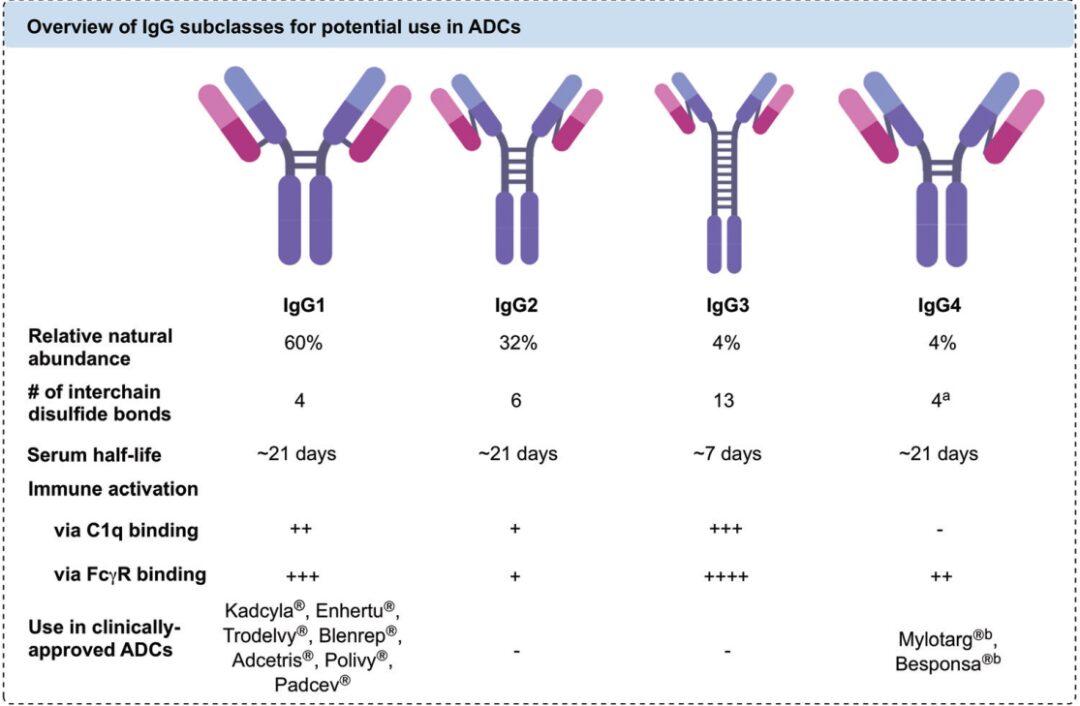
Figure 4: Comparison of Different IgGs
The selection of antibody sources for ADCs has gone through four stages: murine, chimeric, humanized, and fully human antibodies. Due to the high immunogenic rejection, low efficacy, and short circulation half-life of murine and chimeric antibodies, with the development of genetic engineering technology, humanized and fully human antibodies have become the first choice in ADC design, and currently, the vast majority of clinical and preclinical studies use the latter two types of antibodies.
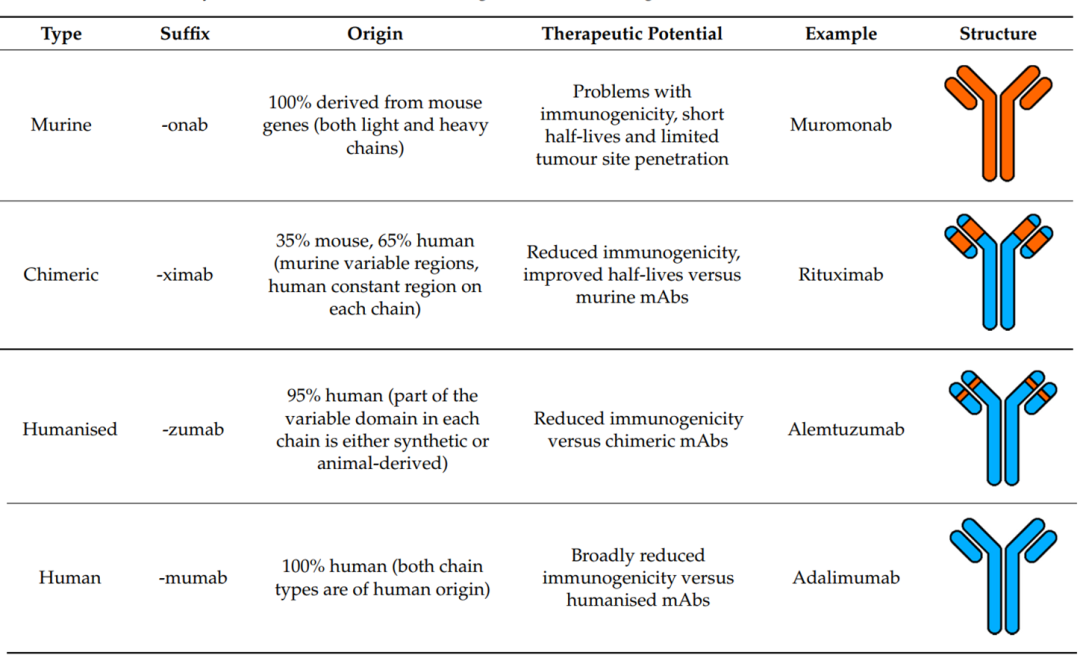
Figure 5: Comparison of Different Antibody Sources (Orange: Murine; Blue: Human)
When selecting antibodies, antibody-dependent cellular cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis (ADCP) should also be carefully considered. ADCC and ADCP mediate the involvement of the immune system in the process of killing tumor cells, affecting the final therapeutic effect of ADCs, so thorough research on the ADCC and ADCP effects of antibodies is essential when designing ADCs.
Selection of Small Molecule Drugs
Early ADC research generally selected traditional small molecule chemotherapeutic drugs, including doxorubicin, but due to the limited number of small molecule drugs that can be conjugated with a single antibody and the limited accumulation of ADCs in tumor tissues, these small molecule drugs with half-maximal inhibitory concentrations (IC50) in the micromolar range do not achieve good therapeutic effects. Currently, when selecting small molecule drugs, there is often a requirement for small molecule drug IC50 values to be as low as nanomolar or even picomolar levels. These small molecule drugs mainly include two categories: microtubule inhibitors and DNA-targeting chemotherapeutic agents.
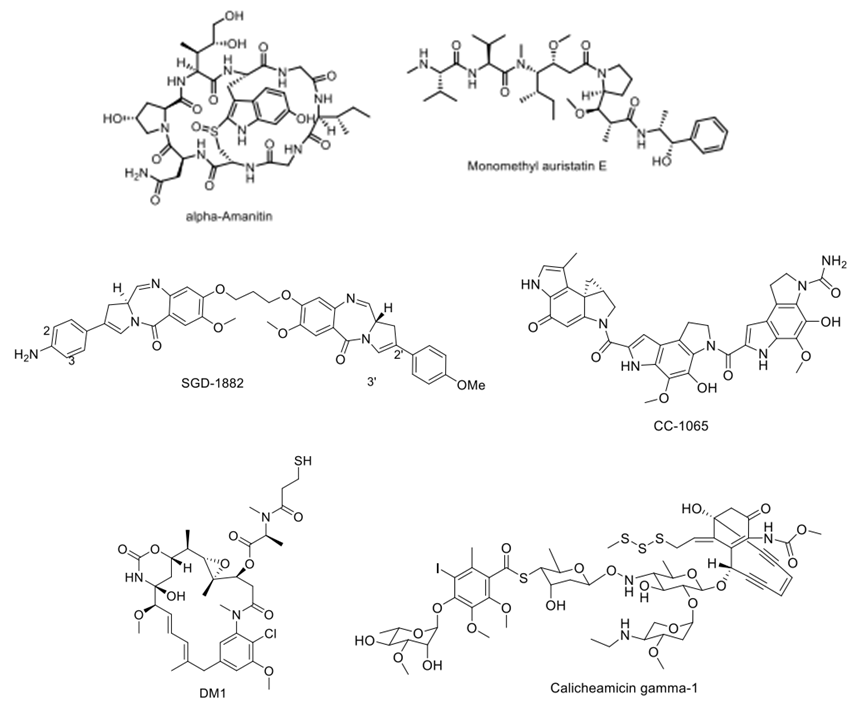
Figure 6: Typical Small Molecule Chemotherapeutic Drugs in ADCs
In addition to requiring a low IC50 value, small molecule drugs usually need to meet the following points: 1. They should not easily cause aggregation of ADCs when conjugated to antibodies to ensure longer circulation time in vivo; 2. Low immunogenicity of both the small molecule drug itself and the formed ADC; 3. Sufficient stability in aqueous solution (blood) and suitable reactive sites for conjugation with antibodies through linkers, while ensuring biological activity after conjugation; 5. They should be able to be synthesized through relatively cost-effective processes.
Selection of Linkers
Linkers can significantly impact the toxicity, stability, specificity, and other properties of the final ADC. The selection of linkers is one of the greatest challenges in ADC development. When choosing linkers, it is essential to ensure that the final ADC can circulate for a long time in the body while remaining stable, and that after entering the cells, the small molecule drug can be released through specific mechanisms to exert further cytotoxicity. Linkers can generally be divided into non-cleavable types used in first-generation ADCs and cleavable types used in second and third-generation ADCs.
Non-cleavable:
Non-cleavable linkers mainly include maleimide and thiol-containing hexanamide linkers, which conjugate small molecule drugs with antibodies by forming amide bonds and thioether bonds. Non-cleavable linkers ensure high stability of ADCs during circulation in the body. After entering cells mediated by antibody-antigen interaction, ADCs are transported into lysosomes, where various biological enzymes degrade the antibody, releasing small molecule drugs to exert toxicity. However, ADCs using non-cleavable linkers can typically only specifically treat tumor cells with high antigen expression, as the small molecule drugs released in lysosomes usually carry charged amino acid residues, which cannot cross the cell membrane and thus cannot kill surrounding tumor cells through bystander effects.
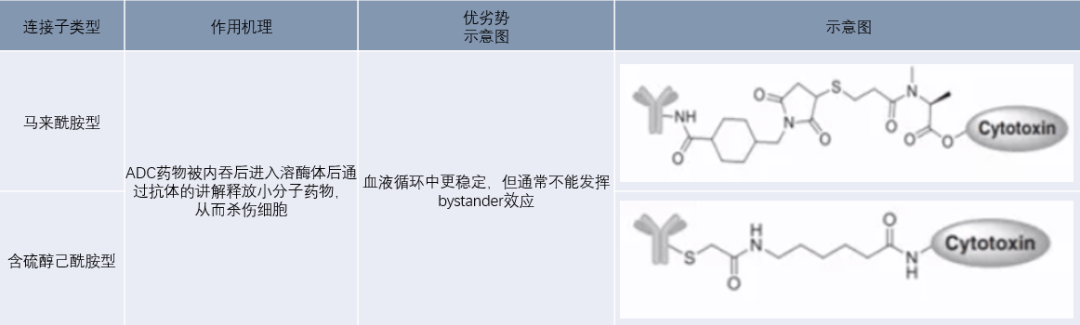
Figure 7: Non-cleavable Linkers
Cleavable:
Cleavable linkers are mainly divided into pH-sensitive, enzyme-sensitive, and reducible types, which utilize the lower pH of tumor tissues, proteases within tumor cells, and the higher reducibility within tumor cells to cleave the linkers. ADCs using this type of linker can be targeted to tumor cells through antibodies, accumulate in tumor regions, and utilize the unique microenvironment of tumor cells to release small molecule drugs, thereby inhibiting cell proliferation and killing cells.
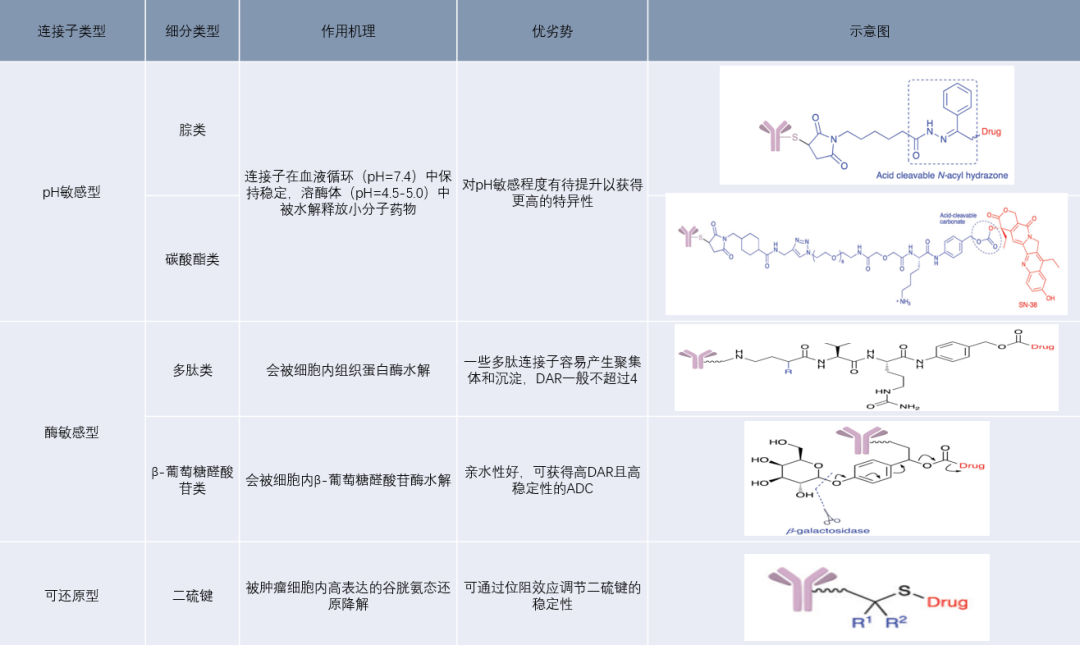
Figure 8: Cleavable Linkers
Drug-Antibody Ratio (DAR)
The number of drug molecules attached to an antibody is defined as the Drug-Antibody Ratio (DAR), which can be obtained through testing methods such as HPLC-MS. A clear DAR value is essential in the later stages of ADC drug development. The number of ADCs taken up by tumor cells during circulation in the body is limited; therefore, a higher DAR is generally favorable for improving efficacy. However, the small molecule drugs used in ADCs tend to be highly hydrophobic, and excessively high DAR values can lead to aggregation of ADCs, resulting in reduced circulation half-life and increased toxic side effects. Thus, excessively high DAR values are undesirable, and the typical DAR range for preclinical and clinical ADCs is between 2-8. To achieve higher DAR and uniformity in ADCs, genetic engineering can be used to modify antibodies to have fixed and efficient reactive sites for conjugating small molecule drugs.
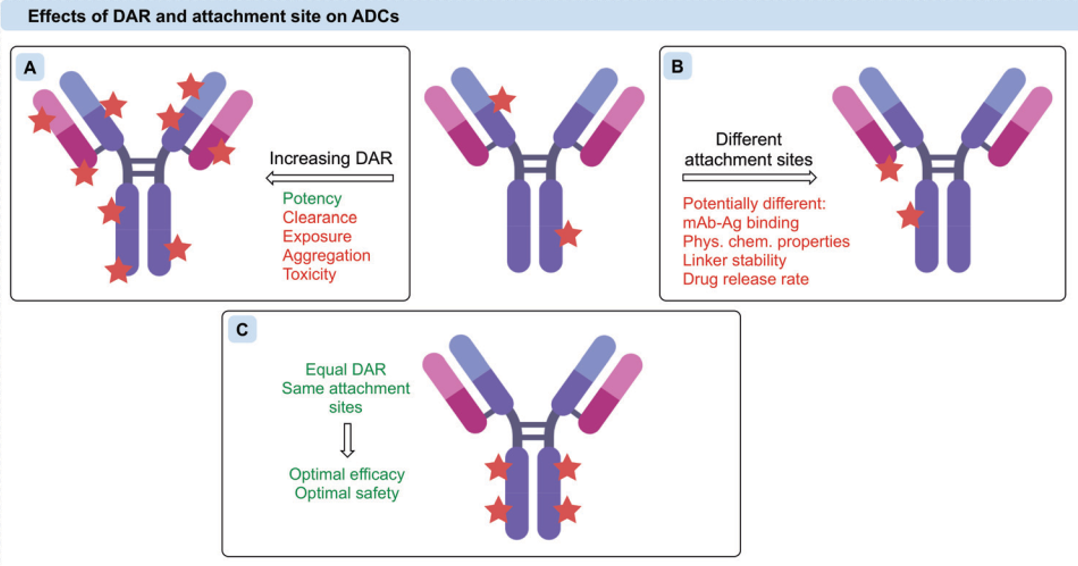
Figure 9: The Impact of DAR on ADC Efficacy
Conclusion
ADC drugs combine the advantages of both antibodies and small molecule drugs, providing a more efficient strategy for cancer treatment. Since multiple ADC drugs have been launched, the market response has been positive, and the ADC drug market is currently vast with a promising future. The final therapeutic effect of ADC drugs is influenced by multiple factors, and the reasonable design and selection of antibodies, small molecule drugs, and linkers in the early stages of ADC development are of utmost importance.
References
Resources:
Reply in the backend:2020, to obtain the Top 200 SMALL Molecule Drugs by Sales in 2020 high-definition structure pdf file.
Reply in the backend:200, to obtain the Top 200 Brand Name Drugs by Retail Sales in 2019 high-definition structure pdf file;
In the backend reply:5, to obtain the Beyond Rule of Five (bRo5) Orally Active Pharmaceuticals high-definition structure pdf file;
Reply in the backend:2018, to obtain the Top 200 SMALL Molecule Drugs by Sales in 2018 high-definition structure pdf file.

