
Organic Electrocatalysis QQ Group: 692419836
Research Background


Amino acids are the basic building blocks of living organisms and have wide applications in food, pharmaceuticals, and the chemical industry. Traditional methods for amino acid synthesis mainly include biological fermentation and chemical synthesis. While biological fermentation is environmentally friendly, it is costly and has a complex purification process; chemical synthesis, although cheaper, requires the use of highly toxic cyanides and energy-intensive production of ammonia. In contrast, electrocatalytic C−N coupling is a more efficient and environmentally friendly strategy for amino acid synthesis, capable of simultaneously removing environmental pollutants and producing nitrogen-containing organic compounds. However, the reaction pathway of electrocatalytic C−N coupling is complex, with intense competition for the adsorption of reactants and intermediates at a single active site, leading to low activity (yield) and selectivity (Faradaic efficiency, FE) for amino acids.
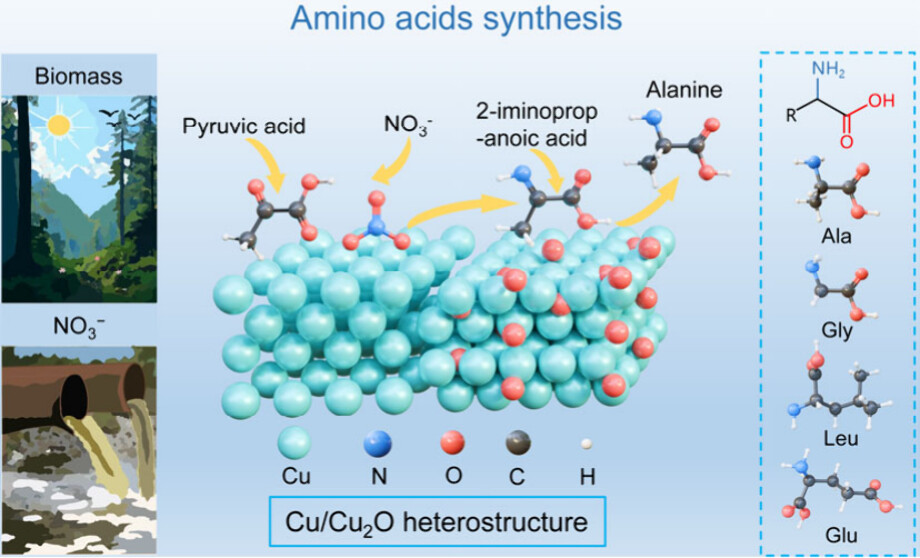
(Image source: J. Am. Chem. Soc.)
Key Points of This Article


This article proposes a cascade catalytic strategy based on Cu/Cu2O heterostructures for the electrosynthesis of amino acids from nitrates and keto acids. By designing dual active sites (Cu0 and Cu+), the adsorption of reactants and intermediates is balanced, thereby optimizing the C−N coupling reaction pathway and improving the yield and selectivity of amino acids.
1. The CuO/C catalyst was synthesized through mild heating and a simple calcination process, and the Cu/Cu2O heterostructure was obtained through an electrochemical reconstruction process. These heterostructures have abundant Cu/Cu2O interfaces that facilitate the transfer of intermediates. Experimental results show that this catalyst can efficiently synthesize various amino acids under mild conditions, including alanine, glycine, leucine, and glutamic acid, with a Faradaic efficiency of up to 75.78% and a yield of up to 478.89 mmol h−1 gcat−1.
2. Through experiments and density functional theory (DFT) calculations, the cascade catalytic mechanism on the Cu/Cu2O heterostructure was revealed. At the Cu0 site, keto acids and nitrates preferentially adsorb and couple to form C−N intermediates, which then migrate to the nearby Cu+ site for protonation, ultimately generating the target product. The abundant Cu/Cu2O interfaces facilitate the transfer of intermediates, increase the reaction rate, and assist the cascade catalysis on the Cu0/Cu+ dual active sites, achieving excellent performance.
Image Analysis


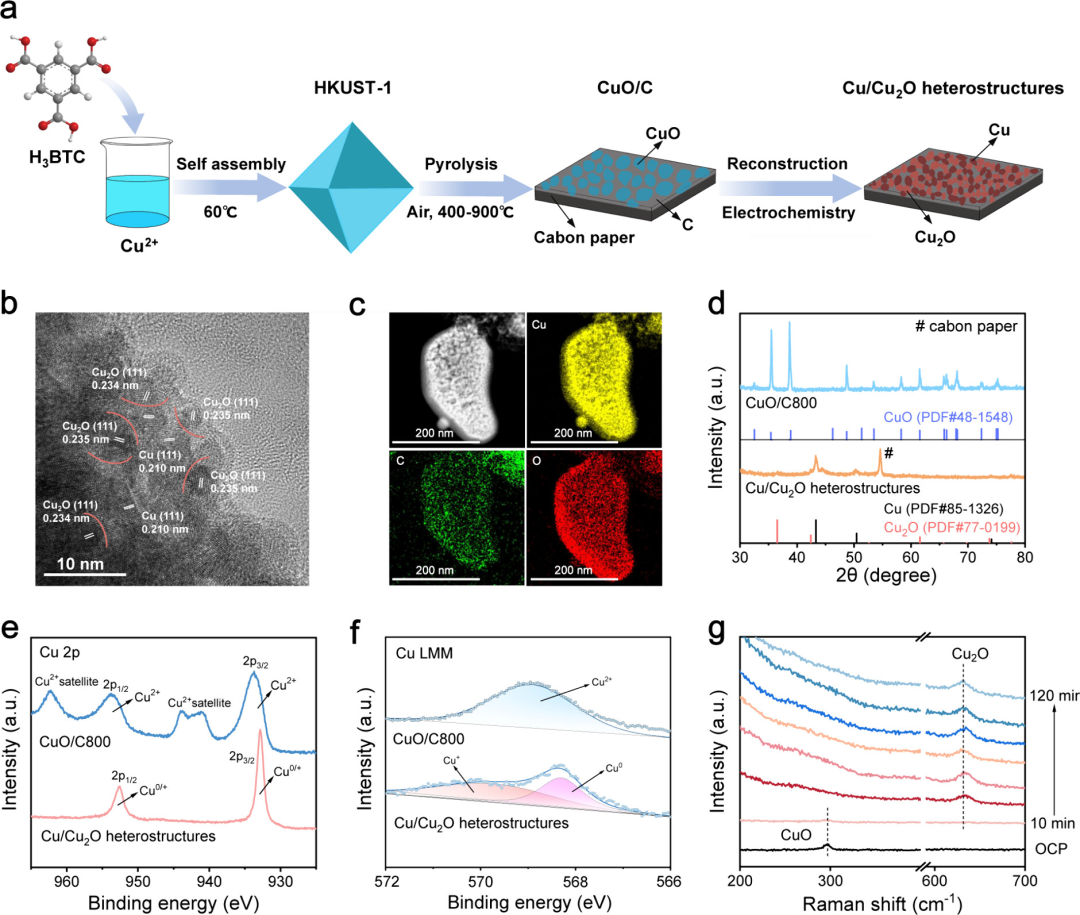
Figure 1: Synthesis and characterization of CuO/C800 catalyst
(Image source: J. Am. Chem. Soc.)
CuO/C catalyst was synthesized through mild heating and a simple calcination process, and the Cu/Cu2O heterostructure was obtained through an electrochemical reconstruction process. High-resolution transmission electron microscopy (HRTEM) images show that the CuO/C800 catalyst was reconstructed into a Cu/Cu2O heterostructure after the electrocatalytic reaction, with abundant Cu(111) and Cu2O(111) interfaces. Energy-dispersive X-ray spectroscopy (EDS) mapping revealed a uniform distribution of Cu, C, and O elements in the catalyst. X-ray diffraction (XRD) and X-ray photoelectron spectroscopy (XPS) analyses further confirmed that CuO was reduced to Cu/Cu2O during the electrocatalytic process. Auger electron spectroscopy (AES) analysis also showed the presence of Cu0 and Cu+ species, indicating that the Cu2O content decreased with increasing calcination temperature. In situ Raman spectroscopy further revealed the structural changes of the CuO/C800 catalyst during the electrocatalytic synthesis of alanine, confirming the transformation from CuO to Cu/Cu2O heterostructure.
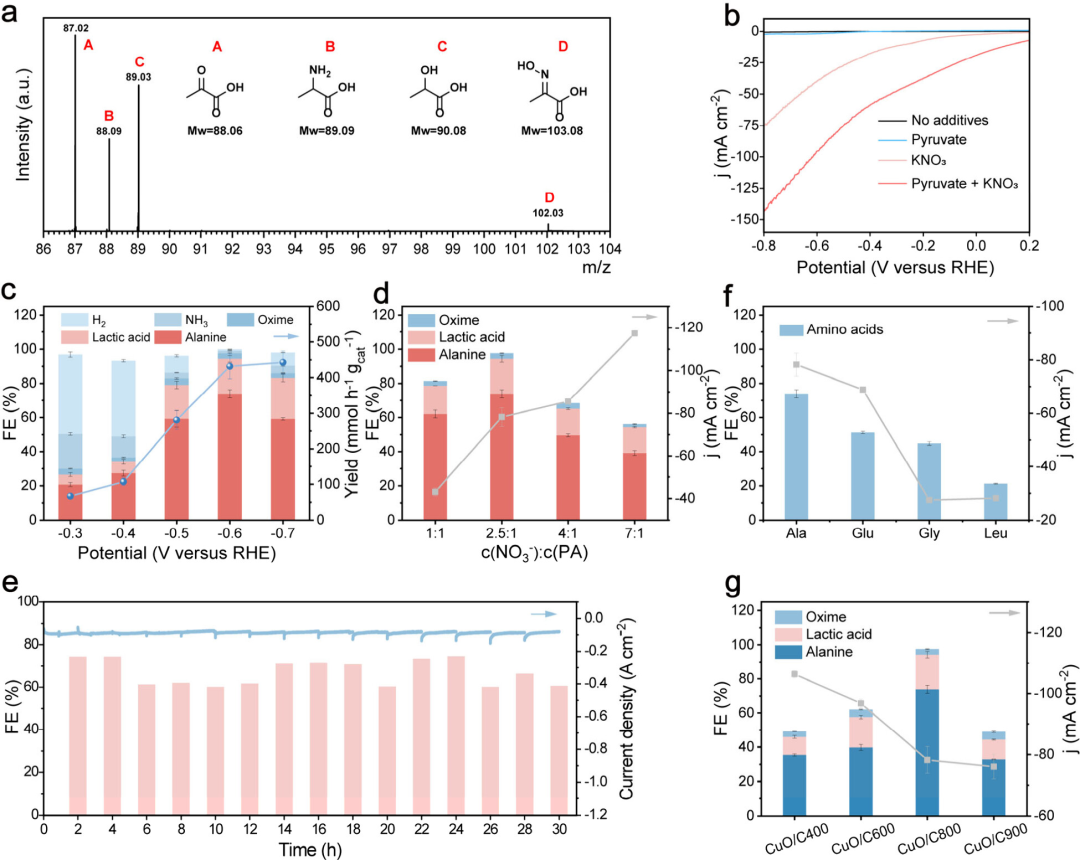
Figure 3: Electrosynthesis performance of amino acids
(Image source: J. Am. Chem. Soc.)
Alanine production was confirmed by liquid chromatography-mass spectrometry (LC−MS), and the structure of the product was further verified by1H NMR spectroscopy. Linear sweep voltammetry (LSV) curves evaluated the electrocatalytic activity of the CuO/C catalyst in different electrolytes, showing that the CuO/C800 catalyst exhibited a significant increase in current density during alanine synthesis. At different potentials, the Faradaic efficiency (FE) and yield of alanine varied with potential, with the highest FE reaching 75.78% and a yield of up to 478.89 mmol h−1 gcat−1. Additionally, the researchers explored the effect of the molar ratio of nitrate to pyruvic acid on alanine yield, finding that a molar ratio of 2.5:1 yielded the highest FE for alanine. Long-term stability tests showed that the CuO/C800 catalyst maintained stable current density and high FE over a continuous 30-hour reaction, demonstrating good catalytic stability.
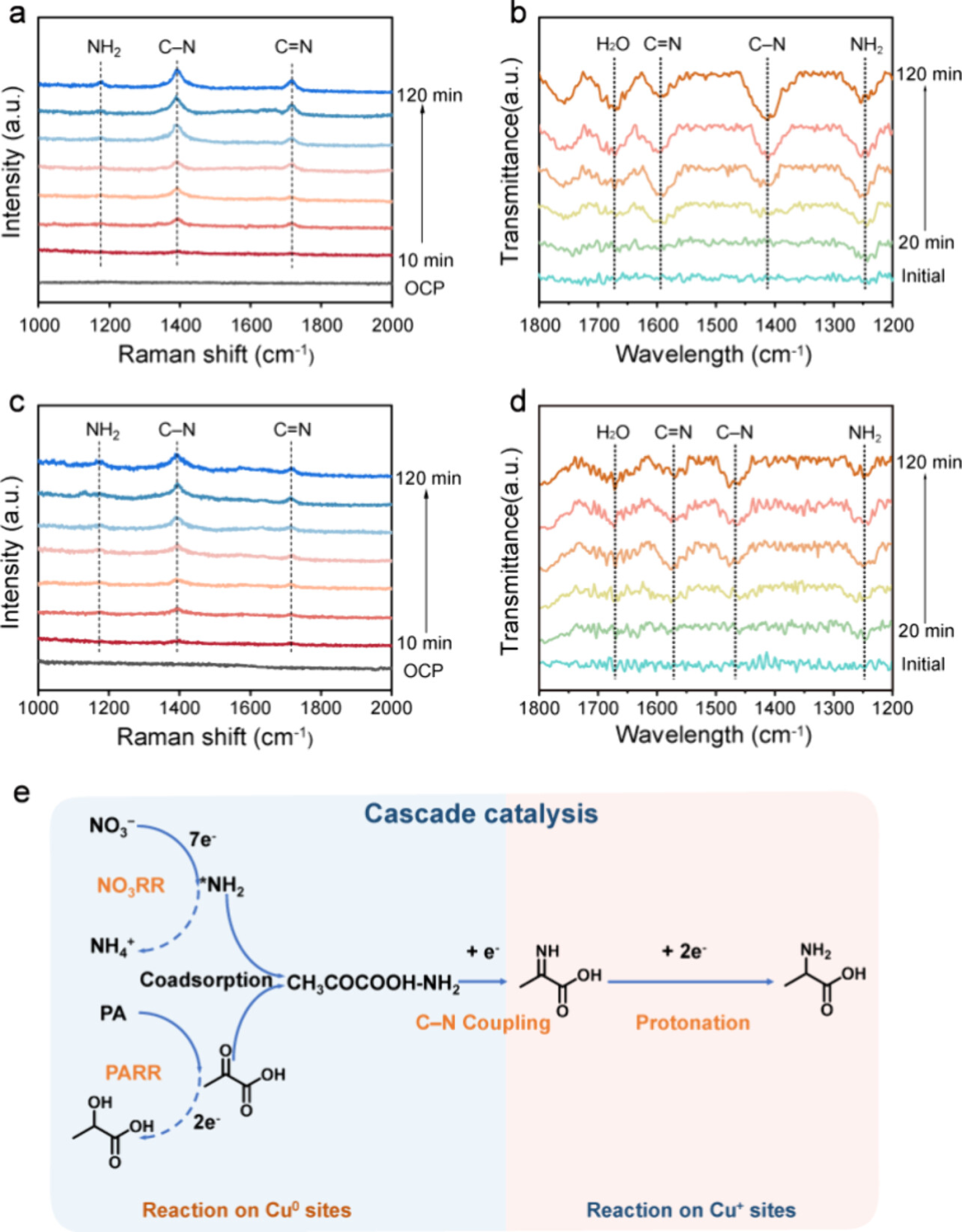
Figure 4: In situ spectroscopy reveals the pathway for alanine generation
(Image source: J. Am. Chem. Soc.)
Time-resolved in situ Raman spectroscopy and synchrotron Fourier-transform infrared spectroscopy (SR-FTIR) monitored the evolution of active intermediates during the synthesis of alanine using the CuO/C800 catalyst. In situ Raman spectroscopy showed that the NH2 vibration peak appeared in the early stages of the reaction, followed by the gradual emergence of C−N and C=N vibration peaks, indicating the formation of C−N bonds and the generation of alanine. SR-FTIR spectroscopy further confirmed the formation of NH2, C−N, and C=N bonds, revealing the C−N coupling reaction pathway of nitrate and pyruvic acid on the Cu/Cu2O heterostructure. These results indicate that the reduction of nitrate and the formation of C−N bonds preferentially occur at the Cu0 site, followed by the migration of intermediates to the Cu+ site for protonation, ultimately generating alanine. The abundant Cu/Cu2O interfaces facilitate the transfer of intermediates, increase the reaction rate, and assist the cascade catalysis on the Cu0/Cu+ dual active sites, achieving excellent performance.
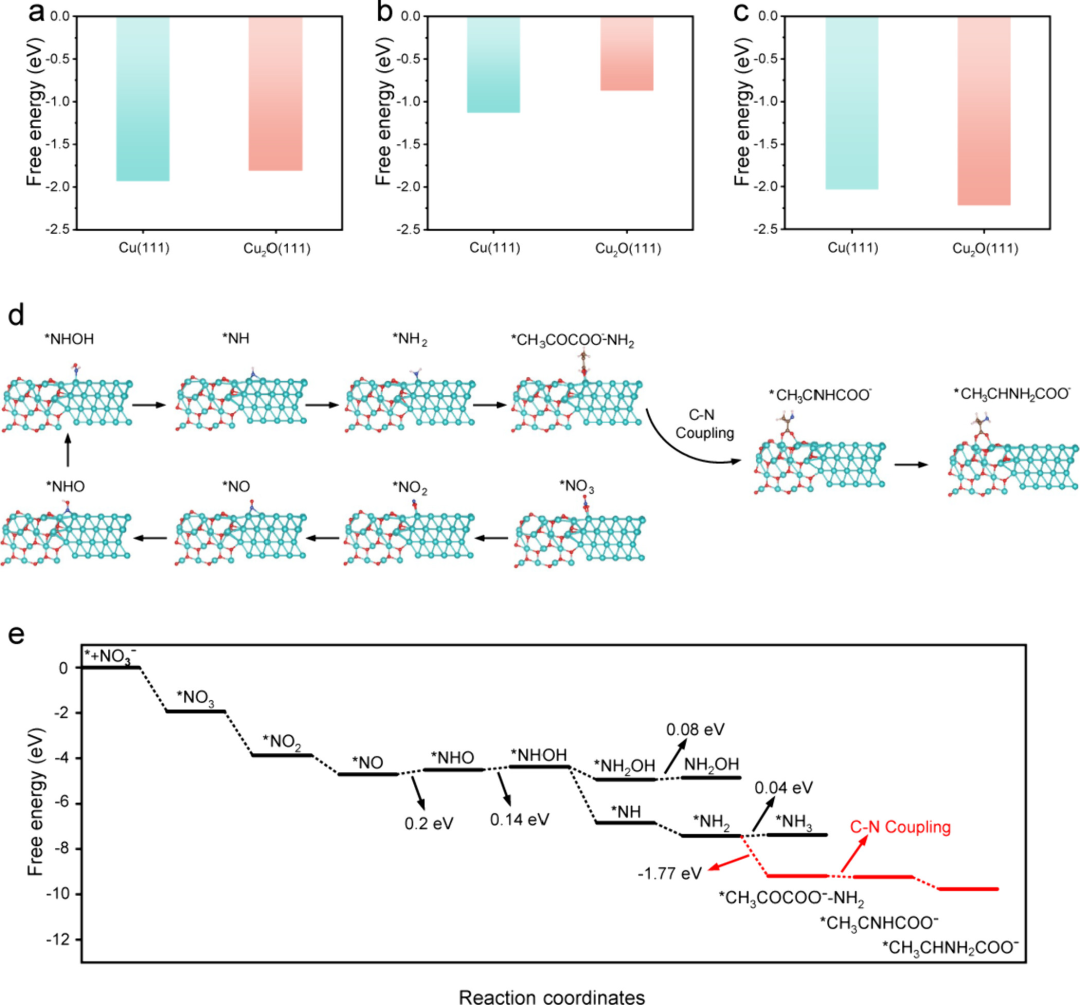
Figure 5: DFT calculations
(Image source: J. Am. Chem. Soc.)
The adsorption free energies of nitrate, pyruvic acid, and alanine intermediates on Cu0 and Cu+ sites were calculated, revealing the cascade catalytic mechanism of the Cu/Cu2O heterostructure in the C−N coupling reaction. The results indicate that the adsorption of nitrate and pyruvic acid on Cu(111) is more favorable, while the adsorption of alanine intermediates is more stable on Cu2O(111). Reaction pathway analysis shows that *NO → NHO and NHO → *NHOH are thermodynamically unfavorable steps, considered to be the rate-determining steps (RDS). In contrast, the generation of *NH2 is thermodynamically more favorable. DFT calculations further reveal that the Cu0 site promotes the activation of nitrate and the formation of C−N bonds, while the Cu+ site accelerates the subsequent protonation steps.
Conclusion and Outlook


This study achieved a cascade catalytic strategy for the efficient electrosynthesis of amino acids from nitrates and keto acids by designing dual active sites on Cu/Cu2O heterostructures. This strategy not only improves the yield and selectivity of amino acids but also provides new insights for designing bimetallic active sites to efficiently construct C−N bonds.
References


Dual Active Sites on Cu/Cu2O Heterostructures for the Cascade Electrocatalytic Synthesis of Amino Acids. J. Am. Chem. Soc. 2025..
DOI: 10.1021/jacs.5c04649
https://doi.org/10.1021/jacs.5c04649

end