
In recent years, new antibody-drug conjugates (ADCs) have entered the public eye with great momentum, becoming the next research hotspot in the field of cancer treatment.
Following chemotherapy, targeted therapy, and immunotherapy, ADCs, which couple cytotoxic drugs with targeted monoclonal antibodies, have opened the fourth ladder of drug treatment for tumors with remarkable achievements. They have become a new class of drugs that cannot be ignored in cancer treatment, including lung cancer, both domestically and internationally.
In the field of lung cancer treatment, in addition to targeted therapy and immunotherapy, ADC treatments related to lung cancer are also increasing.
Antibody-drug conjugates (ADCs) are a class of targeted biopharmaceutical agents that couple monoclonal antibodies with highly cytotoxic drugs through specific linkers. They use monoclonal antibodies as carriers to efficiently deliver small molecule cytotoxic drugs to target tumor cells in a targeted manner.
The concept of ADCs originated from the “magic bullet” concept proposed by Paul Ehrlich 100 years ago, but rapid progress was not seen until the 1980s with the development of non-immunogenic (especially humanized) monoclonal antibodies. ADCs combine the advantages of targeted, highly selective antibodies with highly anti-tumor active cytotoxic drugs, retaining the tumor-killing characteristics of small molecule cytotoxic drugs while selectively reducing the off-target side effects of these drugs, effectively improving the benefit-risk ratio of anti-tumor treatment. Therefore, in recent years, ADCs have been one of the hot research directions in the field of precision cancer treatment.
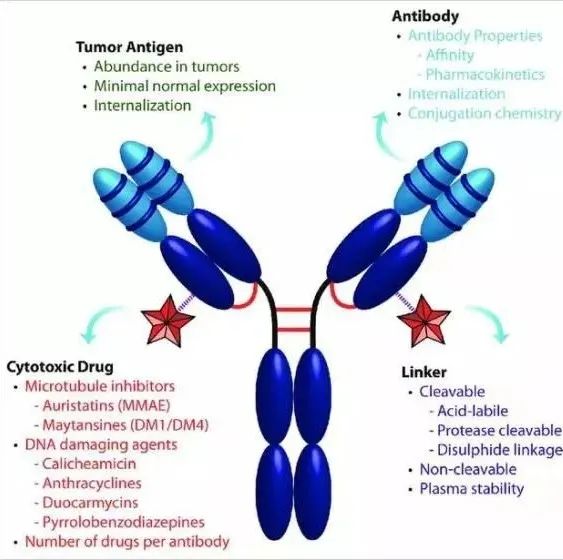
(Schematic diagram of ADC drug structure)
Lung cancer, being the most prevalent and deadliest cancer globally, has seen researchers never cease their exploration for treatment. In addition to targeted therapy and immunotherapy, ADC treatments related to lung cancer are also increasing. Previous studies have shown that ADCs have certain efficacy in treating refractory non-small cell lung cancer (NSCLC), with objective response rates (ORR) reaching 20.3% to 39%.
Currently, there are many ADC targets for lung cancer, including: HER2, HER3, TROP2, CEACAM5, and C-met.
HER2 Targeted ADCs
Unlike other cancers, the abnormal HER2 target in lung cancer manifests in three different types: HER-2 mutations (exon 20), HER-2 amplification, and high expression of HER-2 protein. ADCs have been explored for treatment in these forms.
The results of a phase II basket trial of trastuzumab emtansine (T-DM1) for treating advanced NSCLC with HER-2 amplification or mutations showed that the ORR for patients with HER-2 mutations and amplifications was 44%-50%, with a median PFS of 5-7 months. However, for patients with HER2 overexpression, the ORR was 44% and PFS was 5 months for those with treated advanced lung cancer with HER2 mutations; the ORR was 43% and PFS was 7 months for treating HER2 amplified lung cancer, both showing certain efficacy. However, the efficacy was poor for patients with metastatic NSCLC with HER2 protein overexpression, with an efficacy rate of 0.
Another novel HER-2 targeted ADC, T-Dxd (DS-8201a), showed mid-term results for treating advanced lung cancer with HER2 mutations or overexpression: the efficacy rate was 58.8%, with 72.7% for HER2 mutations. In the phase II DESTINY-Lung01 study, DS-8201 showed an efficacy rate of 61.9% and PFS of 14 months for treating advanced lung cancer with HER2 mutations. The efficacy rate for treated advanced lung cancer with HER2 protein expression was 24.5%, with a PFS of 5.4 months.
TROP2 Targeted ADCs
TROP2 (trophoblast cell surface antigen 2) is a transmembrane glycoprotein that is highly expressed in NSCLC and other solid tumors. TROP2 overexpression is associated with poor prognosis in tumor patients and may be a promising tumor therapy target.
The results of the phase I TROPION-PanTumor01 study in advanced NSCLC patients showed that the antitumor activity of Dato-DXd (DS-1062) (NSCLC dose expansion cohort) had ORRs of 23%, 21%, and 25% for different dose groups; the median PFS was longest in the 6mg dose group, reaching 8.2 months. Based on good efficacy and safety results, 6mg/kg Dato-DXd was chosen for the phase III TROPION-Lung01 (NCT04656652) study, treating advanced or metastatic NSCLC patients who had previously received immunotherapy and platinum-based chemotherapy.
HER3 Targeted ADCs
HER3 is expressed in 83% of NSCLC, but its alteration as a resistance mechanism for EGFRm NSCLC EGFR TKI is not well known. Patritumab-Deruxtecan (HER3-DXd) is an ADC targeting HER-3, currently undergoing clinical research in NSCLC, metastatic breast cancer, and colorectal cancer.
In the phase I study of HER3-DXd for lung cancer, the main participants were those with metastatic/unresectable EGFR mutation NSCLC who progressed after osimertinib or erlotinib, gefitinib, or afatinib, with most patients having undergone platinum-based chemotherapy, and nearly half having received immunotherapy. Among 49 patients diagnosed, the ORR was 25%, and efficacy was observed in patients with different resistance mechanisms detected, with similar remission rates and PFS of 8.2 and 8.3 months for patients with and without brain metastases, respectively. Remission occurred in patients with different HER3 membrane positivity scores at baseline, and the safety profile was controllable, with a low proportion of treatment discontinuation due to adverse events. Currently, a phase II clinical study is ongoing.
CEACAM5 Targeted ADCs
In normal adult tissues, the cancer embryonic antigen-related cell adhesion molecule 5 (CEACAM5) is located in the stomach, tongue, esophagus, cervix, sweat glands, and prostate. After malignant transformation, the cancer embryonic antigen can be detected in ad-NSCLC, SCLC, pancreas, gallbladder, bladder, ovarian mucinous carcinoma, endometrial, colorectal cancer, and gastric cancer.
SAR408701 is a CEACAM-targeted ADC, with preliminary efficacy and safety reported. In a phase I study design expansion phase, the ADC drug SAR408701 was used to treat previously treated advanced non-squamous NSCLC patients, showing an efficacy rate of 20.3% in patients with high CEACAM5 expression. SAR408701 has shown promising antitumor activity in previously treated, high CEACAM5-expressing advanced non-squamous NSCLC patients, with good tolerability and lower hematologic toxicity compared to traditional chemotherapy drugs. Currently, a phase III clinical trial (CARMEN) is underway in advanced non-squamous NSCLC patients who have failed first-line chemotherapy or immunotherapy, based on preliminary efficacy and tumor cell IHC showing CEACAM5 ≥50%.
MET Targeted ADCs
Telisotuzumab Vedotin (ABBV-399) is an ADC targeting MET amplification and c-Met overexpression tumors.
The efficacy of ABBV-399 in lung squamous cell lung cancer patients was evaluated in the LUNGMAP sub-study S1400K, showing an ORR of only 9% among 23 re-treated patients, with a median PFS of 2.4 months and median OS of 5.6 months, leading to the cessation of further research in lung squamous cell carcinoma patients.
In a phase II study of ABBV-399 in previously treated c-Met+ NSCLC subjects, an ORR of 35% was observed in EGFR wild-type non-squamous cancer patients, with a median duration of response (mDOR) of 6.9 months, where the ORR was as high as 54% in high-expression patients. Currently, a phase III clinical trial comparing ABBV-399 with docetaxel in c-MET expressing patients is ongoing.
The development of new ADC drugs provides a new possibility for the treatment of lung cancer patients, enriching clinical treatment options, especially with the hope of breakthroughs in HER-2 and c-MET targets. Future clinical research on ADC drugs will focus more on combination therapies, especially with immune checkpoint inhibitors (ICIs), hoping to achieve breakthroughs.
It is hoped that with the continuous deepening and innovation of ADC drug development, the challenges of treating difficult or resistant lung cancer will be addressed.
(This article is compiled from related online articles)
Editor | Xiao Wei; Layout | Xiao Jian
Some images and texts are sourced from the internet; if there are any objections or infringements, please contact for deletion.
This material is for exchange reference only; if you wish to know more related information, please consult a professional medical worker or visit a regular hospital for diagnosis.
Submissions/Cooperation: [email protected], WeChat 18500776078


