On September 10, 2022, updated data from the open-label, multicenter Phase II DESTINY-Gastric02 study were presented at the ESMO conference, showing that DS-8201 demonstrated sustained clinical benefits and acceptable safety in patients with locally advanced or metastatic HER2-positive gastric cancer or gastroesophageal junction (GEJ) cancer who had previously received trastuzumab-based therapy.
The study included 79 Western patients, with each patient receiving a standard dose of 6.4 mg/kg Q3W. The cutoff date for the updated data was November 8, 2021, with specific information as follows:
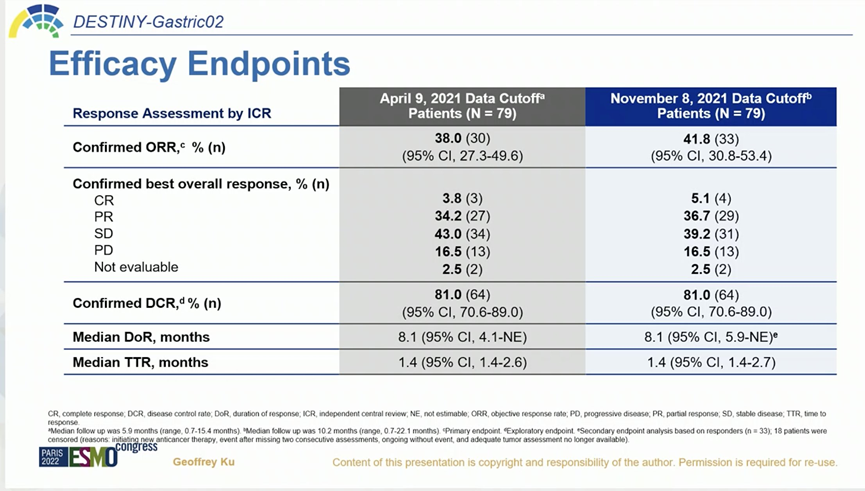
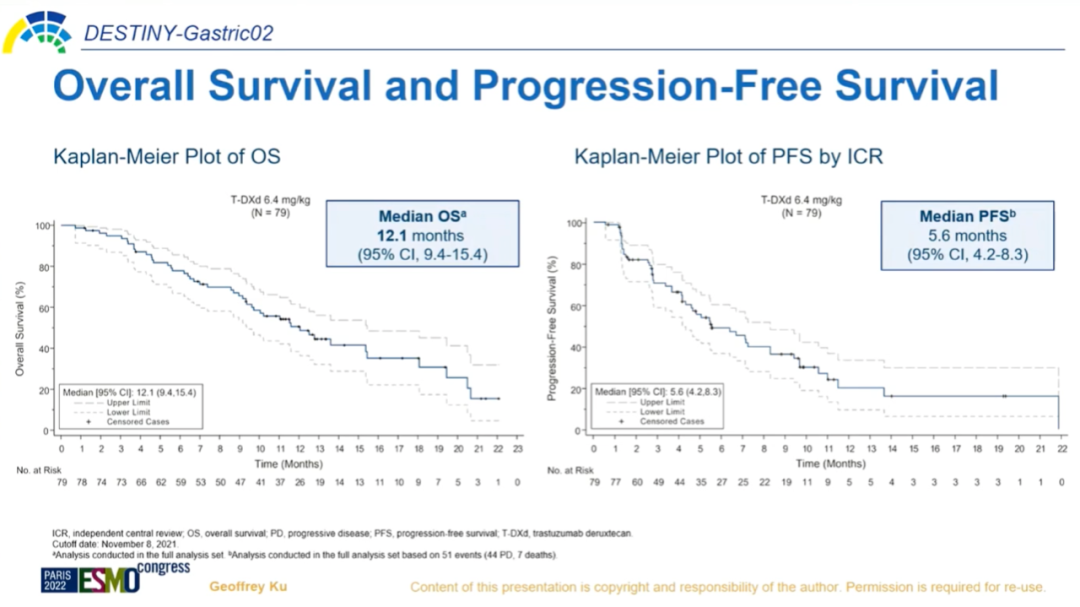
Image Source: Arndt Vogel
The safety profile remained consistent with previous findings. Notably, the incidence of drug-related ILD/pneumonitis was 10.1% (8/79), and two patients died due to severe ILD/pneumonitis. Overall, this study reconfirmed the clinical benefits and acceptable safety of DS-8201 in the Western population, further advancing the standard of care for second-line HER2-positive gastric cancer.
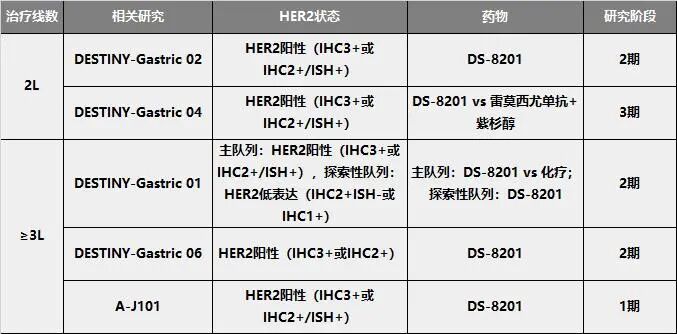
DS-8201 Second and Third-Line Research Layout
Currently, the CSCO guidelines in China recommend that second-line standard treatment for HER2-positive advanced gastric cancer primarily consists of anti-angiogenic drugs combined with chemotherapy or single-agent chemotherapy. In the United States, the NCCN guidelines have included DS-8201 as a treatment option for HER2-positive advanced gastric cancer patients who have previously received trastuzumab in the second line and beyond. The European ESMO guidelines also recommend DS-8201 for second-line and later treatment for patients who progressed after first-line treatment with trastuzumab.

Although DS-8201 has not yet been approved in China, there is a significant unmet need in this area, as previous treatments such as trastuzumab, TKI, and T-DM1 have not improved survival outcomes for patients with HER2-positive advanced gastric cancer. More mature Chinese data is needed for DS-8201 to fill this gap.
It is worth mentioning that the ongoing multicenter DESTINY-Gastric 04 study is comparing the efficacy and safety of DS-8201 monotherapy versus paclitaxel combined with ramucirumab (as shown in the image 1A class).
Additionally, the 2022 CSCO guidelines have introduced anti-HER2 ADC therapy for the first time in the third line, recommending Rongchang Biologics’ vidicizumab (RC48) as a level II recommendation 2A class, while continuing to monitor the positive progress of DS-8201.
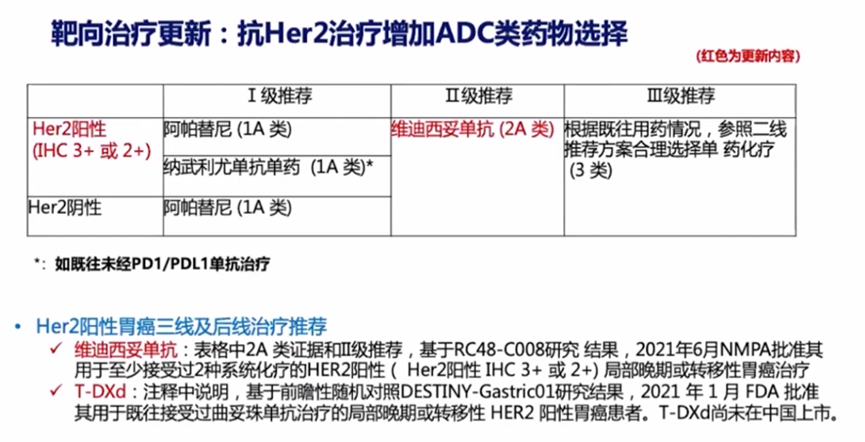
Although the guidelines primarily recommend apatinib or nivolumab for third-line treatment of HER2-positive advanced gastric cancer, the former has a median OS of 6.5 months and an ORR of only 2.8%; the latter has a median OS of 5.3 months and an ORR of 11.2%. In contrast, these two ADCs have provided significant survival benefits for HER2-positive advanced gastric cancer. Previous studies, DESTINY-Gastric01 and C008, have sufficiently supported the guidelines to prioritize these two ADCs, and as Chinese clinical data matures, their recommendation priority may further increase.
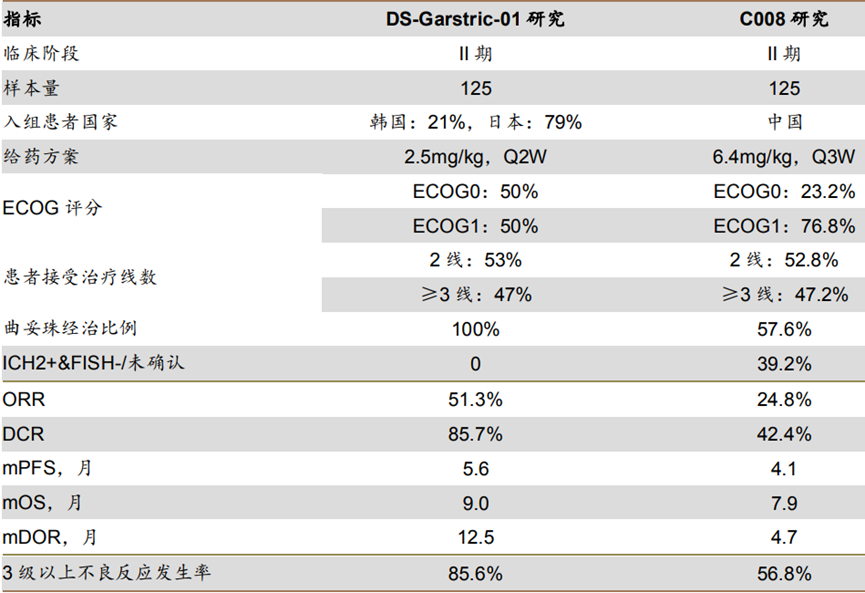
Note: Non-Head-to-Head
 Scan to check available cell lines
Scan to check available cell lines
References: 1. Three Major Gastric Cancer Treatment Guidelines 2022 Edition; 2. Rongchang Biologics Prospectus; 3. https://m.medsci.cn/article/show_article.do?id=6781e3907456
Previous Recommendations
Heidelberg Third-Generation ADCs
Junshi Biosciences Releases Semi-Annual Report, Domestic PD-1 Sales Overview
New Targets for Solid Tumors: CDH17
Click below “Pharmaceutical Research Network” for more exciting content