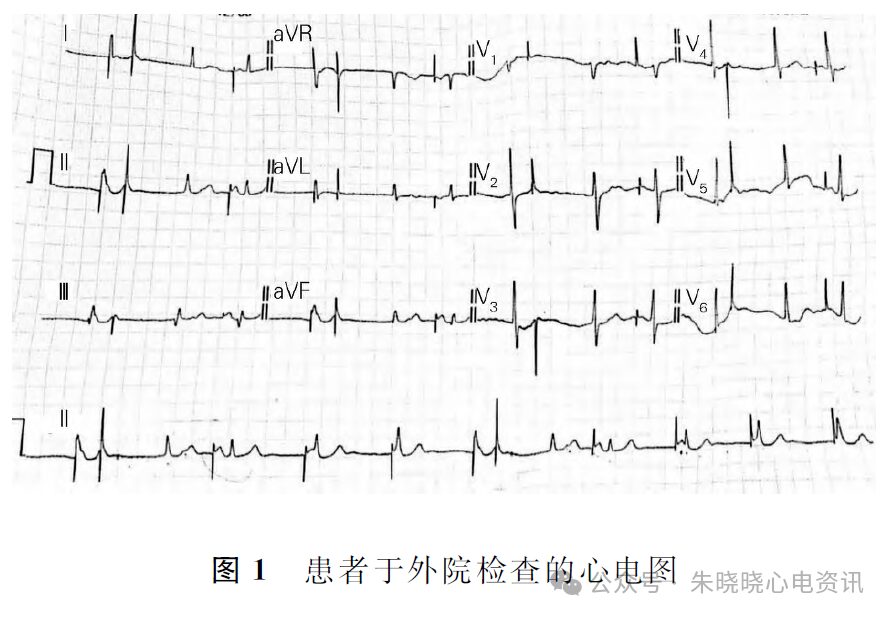
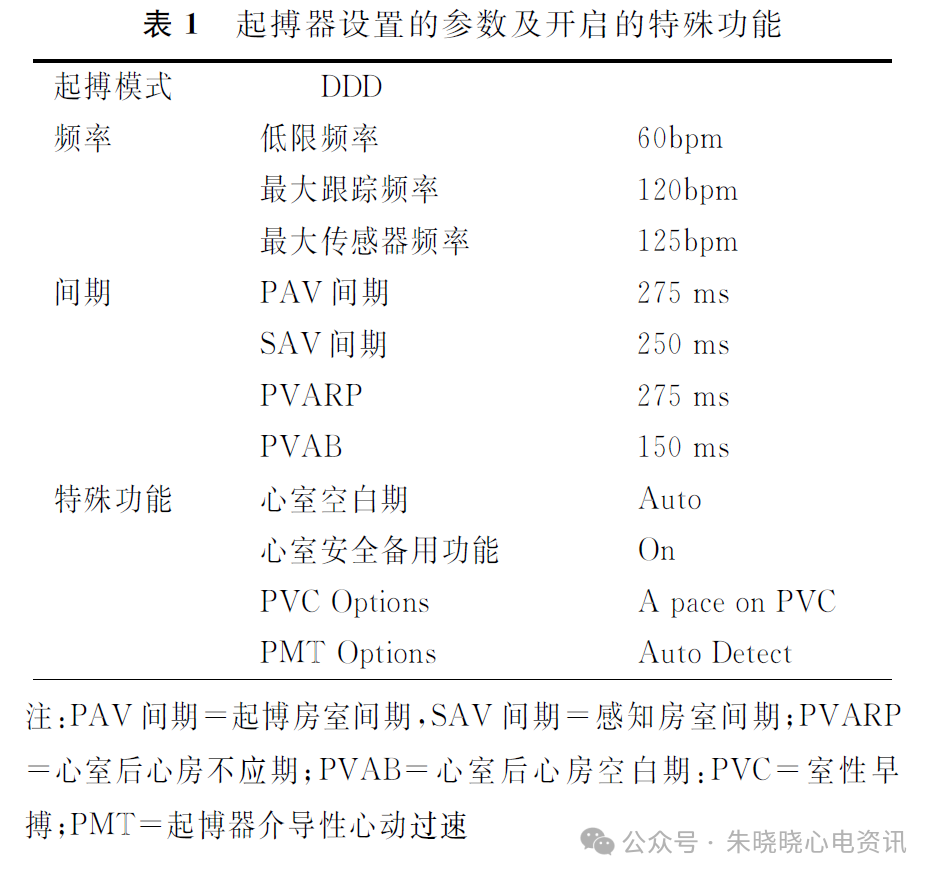
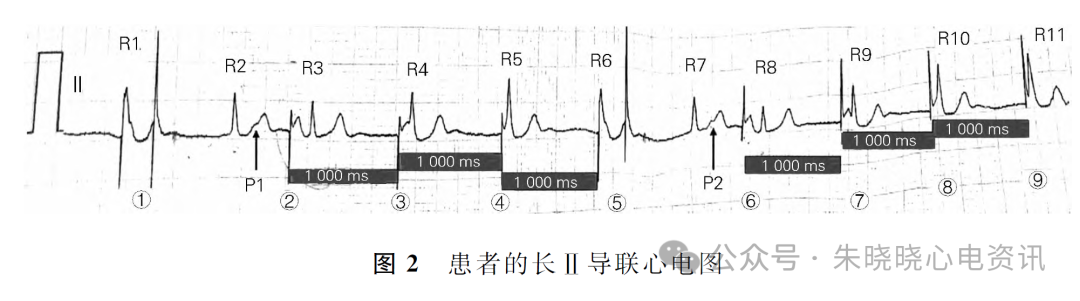
Competition Between Intrinsic Rhythm and Pacemaker Rhythm Leading to Misdiagnosis of Pacemaker Dysfunction
Guo Xiaoyu, Fan Xiaohan, Fan Shuxin
Wangjing Hospital, China Academy of Chinese Medical Sciences
Fuwai Hospital, Chinese Academy of Medical Sciences