Jianggroup@BNU
Suprachem/Topological Chirality

Direct π-extension of a Conjugated Carbon Nanohoop Using a Zipper Method
Abstract: The π-extension of carbon nanohoops to ultra-short carbon nanotubes (CNTs) poses a significant challenge for synthetic chemists. Here, the authors report the synthesis, characterization, and properties of nano-graphene embedded carbon nanohoops (NECR) synthesized via a direct zipper method. In this approach, long linear phenyl chains are fused to the CPP framework through a simple Scholl reaction, akin to connecting two pieces of fabric with a zipper. Its photophysical properties were studied using UV-Vis spectroscopy and photoluminescence spectroscopy. Furthermore, the potential applications of NECR in electronic transport devices were investigated.
Highlight 1: In terms of synthesis, the authors synthesized the extended nano-graphene embedded carbon nanohoop (NECR) using the zipper method through the Scholl reaction, which represents the sidewall portion of [11,11]SWNTs. The specific synthesis is illustrated in Figure 2. First, boronic ester 1 and compound 2 were coupled via Suzuki coupling to obtain the macrocyclic precursor 3, which was then reduced and aromatized using H2SnCl4 to yield macrocycle 4. Next, compound 5 was coupled with macrocycle 4 to form macrocycle 6. Finally, in dry dichloromethane, macrocycle 6 was treated with 12 equivalents of 2,3-dichloro-5,6-dicyanobenzoquinone (DDQ) and trifluoromethanesulfonic acid at 0°C to produce a large π-extended graphene sidewall. After 1 hour of reaction, the final product 7 (NECR) was obtained as a red powder with a yield of 40%.
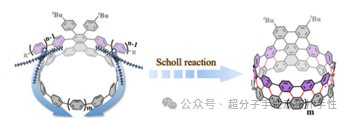
Figure 1. Zipper method for obtaining ultra-short armchair carbon nanotubes.
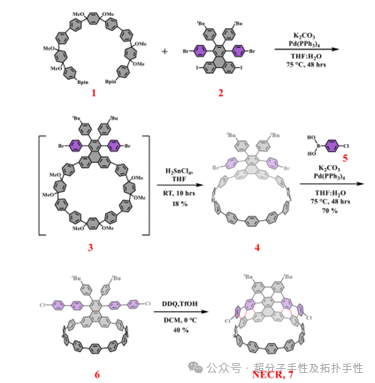
Figure 2. Synthesis steps of NECR.
Highlight 2: To explore the photophysical properties of NECR, its UV-Vis absorption, fluorescence emission spectra, and time-resolved fluorescence decay were studied. Precursor compound 6 was used as a reference compound. As shown in Figure 3a, the absorption spectrum of NECR in CH2Cl2 exhibits two broad absorption bands in the range of 300-580 nm. One absorption band shows three main peaks at 355 nm, 382 nm, and 402 nm. The other band has two absorption peaks at 492 nm and 530 nm. Under excitation at 402 nm, the fluorescence emission spectrum of NECR at room temperature was investigated. An emission band was observed in the range of 500-700 nm, with two emission peaks at 547 nm and 580 nm (Figure 3b). The absorption band of precursor 6 shows a maximum absorption peak at 344 nm. The fluorescence emission spectrum shows two emission peaks at 440 nm and 463 nm (Figure 2b). Notably, the absorption and fluorescence spectra of NECR exhibit a significant red shift compared to those of the precursor, consistent with the extended π-conjugation. The fluorescence quantum yield (QY) of NECR was measured to be ΦF= 80% using an integrating sphere, while the QY of compound 6 was ΦF= 65%. Under UV light at 365 nm, the fluorescence colors of 6 and NECR were blue and bright yellow, respectively. Under excitation at 390 nm, the luminescence lifetime of NECR at 547 nm was determined to be 3.3 ns through single exponential decay fitting (Figure 3d). For precursor 6, a similar single exponential decay was observed at 444 nm under the same excitation wavelength, with a luminescence lifetime of 3.2 ns (Figure 3c).
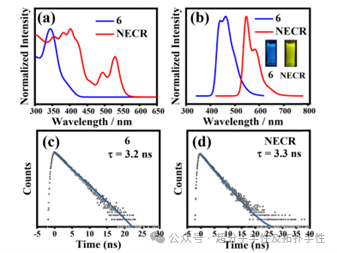
Figure 3. (a) UV-Vis absorption and (b) fluorescence emission spectra of 6 (green) and NECR (red) in CH2Cl2 (1.0×10-5 M). Inset: Fluorescence images of 6 (left) and NECR (right) under 365 nm lamp in CH2Cl2. (c) and (d) Emission lifetimes of 6 and NECR in CH2Cl2.
Highlight 3: In organic electronics, charge transport properties are crucial characteristics of organic materials. Investigating and improving the charge transport properties of conjugated small molecules and polymers is both interesting and meaningful. Due to the high overlap of π-electrons from adjacent molecules, charge carrier mobility can be enhanced, making nano-graphene a promising candidate for the active layer in organic field-effect transistors (OFETs). The authors further investigated the charge transport properties of NECR. It can be used as the active layer in OFETs with a bottom-gate bottom-contact (BG-BC) structure. This BG-BC structure is based on clean SiO2, with Cr/Au deposited on its surface as source/drain electrodes via thermal evaporation. The active layer was drop-cast onto the source/drain electrodes. The devices were annealed at 150°C for 1 hour in an N2 environment. The carrier transfer characteristics of NECR are shown in Figure 4b. The calculated mobility μe of NECR was found to be 9.08×10-5 cm2 V-1s-1, which is higher than the reported mobilities of cyclic oligo-carbazole and nanohoop derivatives, likely due to the larger π-conjugation of NECR.
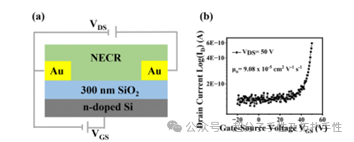
Figure 4. (a) Schematic diagram of BG/BC OFET structure. (b) Transfer characteristics of BG/BC FET devices based on NECR.
Conclusion: In summary, a large π-extended nano-graphene embedded carbon nanohoop was successfully constructed by connecting non-fused phenyl chains to the CPP framework via the zipper method using the Scholl reaction. The target product was confirmed by NMR and mass spectrometry. Steady-state spectroscopy revealed its unique photophysical properties. NECR also exhibited n-type OFET characteristics, with a field-effect mobility reaching 9.08×10-5 cm2V-1s-1. This cyclic fused zipper strategy may provide a possible route for the construction of ultra-short armchair carbon nanotubes.
Due to the limited level of the editor, any inaccuracies are welcome for criticism and correction.
References
Reference Title: Direct π-extension of a conjugated carbon nanohoop using a zipper method
Reference Authors: Kang Wei, Zaitian Cheng, Xinyu Zhang, Qiang Huang and Pingwu Du
https://doi.org/10.1039/d4cc04976d
Copywriter: Fan Shimin
Editor: Guo Shengzhu