
Developing an entry-level ADC seems not difficult, but evaluating the pharmacology and toxicology in the preclinical stage has become a top priority.
Although monoclonal antibody experiences can be referenced, given that ADCs have unique characteristics distinct from monoclonal antibodies, there are many challenges in the research and evaluation of ADCs. The difference between ADCs and naked monoclonal antibodies is that ADCs must bind to and internalize into tumor cells, thereby transporting and releasing small molecule drugs into the cells. The antibody, small molecule drug, linker, and the methodology of linking can all affect the ADCs efficacy and safety.
Currently, there are many in vivo and in vitro non-clinical research methods, and there is also development experience from marketed drugs, but predicting clinical efficacy and safety through optimized non-clinical research remains challenging. This article will conduct an in-depth analysis of this.
The Development History of ADCs
The ideal ADC should at least possess the following three characteristics:
1. Target Binding Ability: The ideal ADC target should be an antigen expressed only on the surface of tumors; it should be expressed at a high level and not easily shed.
2. Internalization Process: Before reaching the target cells, the cytotoxic molecule should shed as little as possible, meaning that the complete ADC should be internalized into the cells.
3. Release of Toxic Small Molecules: Small molecules should be released rapidly and in large quantities only inside tumor cells; small molecules should possess high toxicity to efficiently kill tumor cells. Based on the above characteristics, ADCs have undergone several stages of evolution (see Figure 1).
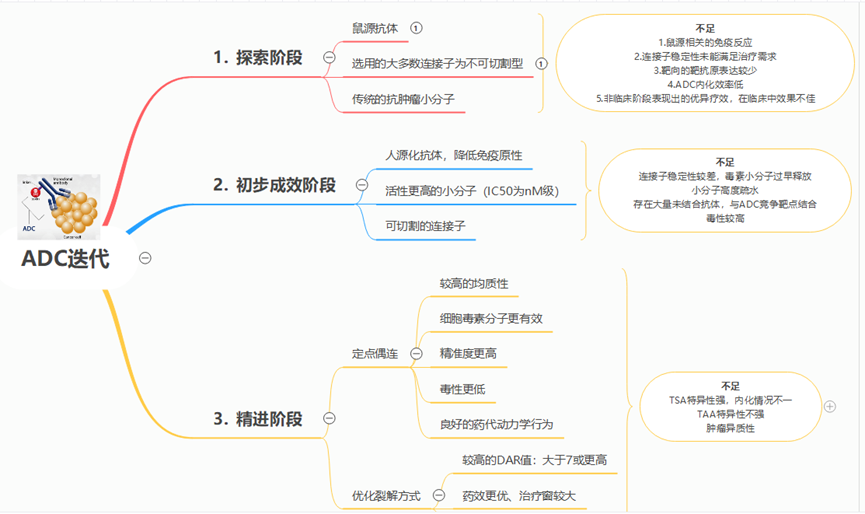
Figure 1 Iteration and Evolution of ADCs
Pharmacological Evaluation Strategies for ADCs
Studies on pharmacological effects usually focus on drug discovery or non-clinical evaluation stages, and the main experimental items that need to be conducted include: target binding activity, antibody-mediated internalization, in vivo and in vitro tumor proliferation inhibition (for anti-tumor ADCs), antibody-dependent cell-mediated cytotoxicity (ADCC), and complement-dependent cytotoxicity (CDC).
In experiments, attention should be paid to the pharmacological differences between ADCs and naked monoclonal antibodies, as well as non-conjugated cytotoxic drugs. In vitro target binding studies play an important role in optimizing linker/linkage methods in the early stages of drug research, as well as selecting relevant animal species for in vivo administration tests. In the early drug optimization screening, attention needs to be paid to the internalization of ADCs; the expression level of target antigens and the heterogeneity of ADCs may both affect the internalization of ADCs.
Typically, immunofluorescence techniques are used to observe the internalization of ADCs in cells and their cross-linking with lysosomes.
In pharmacological effect studies, ADCC and CDC effects also need to be considered. The absence of effect function can have advantages to some extent; the binding of ADCs to effector cells may reduce their accumulation at tumor sites, hindering their internalization and causing normal cytotoxicity. Pharmacodynamic studies conducted at the cellular level or in animal models are the most direct reflections of the pharmacodynamic effects of ADCs.
The expression level of target antigens is a factor that needs to be considered in in vitro or in vivo pharmacodynamic tests. In tumor therapy, even if the target antigen is tumor-associated, if the expression level is not high, it may affect the binding and internalization of ADCs with the target antigen, thereby affecting the targeted transport and release of cytotoxic drugs. The expression level of target antigens is significantly correlated with clinical efficacy.
The FDA approved Kadcyla can significantly improve the progression-free survival and overall survival of patients with high expression of Her2 breast cancer. The progression-free survival (PFS) of patients with high expression of Her2 is 10.6 months, while the PFS of patients with low expression is 8.2 months. The overall survival of high-expression groups is 34.1 months, while that of low-expression groups is 26.5 months.
Typically, immunohistochemical methods are used to confirm the expression level of target antigens in tumor or normal cells; however, immunohistochemistry has the drawback of being difficult to predict immune responses under physiological conditions. In vivo biodistribution or whole-body imaging tests in animal models can provide a comprehensive and intuitive understanding of the expression of target antigens throughout the body, the binding and internalization of ADCs and antigens, and the distribution ratio of tumor tissues to other tissues.
When investigating the pharmacodynamic effects of ADCs in animals, species differences must first be considered. If ADCs have no binding activity or low binding activity with mouse antigens, it may be necessary to use transgenic animals for pharmacodynamic tests or to conduct in vivo pharmacodynamic studies using animal-derived antibodies. Transgenic animals can avoid the use of surrogate antibodies; when the target antigen is expressed in the host’s blood vessels or stroma, transgenic animals can not only evaluate pharmacodynamic effects but also observe toxic reactions to some extent. If the target antigen is expressed only in human tumor cells, there is no need to use transgenic animals; pharmacodynamic effects can be evaluated in nude mouse models implanted with human tumor cells.
In conducting pharmacodynamic studies at the cellular level or in animal models, PK/PD studies should also be conducted to analyze the targeted distribution of the drug, receptor occupancy, and the relationship between drug exposure and effect, which is beneficial for the design of clinical dosing regimens and the analysis of safety study results.
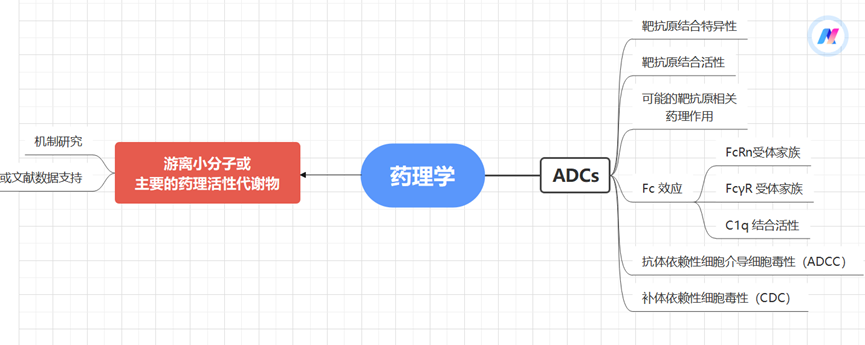
Figure 2 Pharmacological Evaluation Strategies for ADCs
Toxicological Evaluation Strategies for ADCs
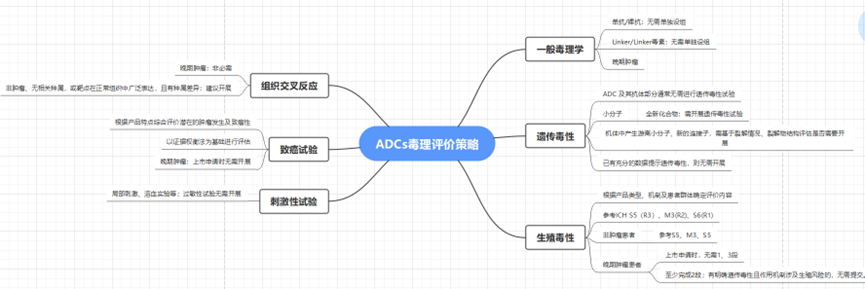
Figure 3 Non-Clinical Evaluation Strategies for ADCs
Selection of Animal Species: In order to adequately expose the toxic characteristics of ADCs in non-clinical safety studies to support the clinical development of ADCs, it is crucial to assess the relevance of the animal species used in trials and the translatability of non-clinical research results. This determination usually involves examining the binding affinity of ADCs to target antigens in non-clinical species compared to humans, as well as comparing immunohistochemical staining patterns in tissue cross-reactivity studies. The effector function or immunogenicity of antibodies also varies due to species differences.
Exposure Parameters and Metabolism: Additionally, as with other drugs and biological products, extrapolation of non-clinical research results to humans should include comparisons of exposure parameters and metabolism. For example, BR-96, which is an ADC covalently linked to human-derived BR96 monoclonal antibody’s cysteine residue via a 6-maleimidohexanoic acid hydrazone linker, showed that the toxic effects of BR-96 were more sensitive in dogs than in rats and monkeys, with hemorrhagic enteritis toxicity being dose-limiting. Surprisingly, BR96-DOX and unmodified monoclonal antibody BR96 exhibited the same dose-limiting toxicity, indicating that the toxicity was generated by the monoclonal antibody. Unlike unconjugated DOX, ADCs did not induce cardiomyopathy in rat models. Therefore, the dose selection for the phase I clinical trial of BR96-Dox in human subjects was based on the toxicological results in dogs.
Expression of Antibody Targets: Similar to therapeutic monoclonal antibodies, the non-clinical evaluation of ADCs may be hindered by the lack of antigen targets in the animal species used for testing. The ICH S6 (R1) guidelines for the preclinical safety evaluation of biotechnological derived drugs (2011) state, “The selection of species for antibody-drug/toxin conjugates (ADCs) containing novel toxins/toxic agents should follow the same general principles as for unconjugated antibodies. The relevant animal species for monoclonal antibody testing should express the required epitopes and display a tissue cross-reactivity profile similar to that of human tissues.” Therefore, the specificity of species and disease may determine or limit the non-clinical evaluation.
An example is Polatuzumab vedotin, which binds to CD79b on human B cells but was found not to bind to mouse, rat, or cynomolgus monkey CD79b, lacking pharmacologically relevant non-clinical species. Non-clinical toxicity studies were conducted using surrogate ADCs that bind to cynomolgus monkey CD79b with affinities similar to Polatuzumab vedotin and human CD79b, which is another method described in the ICH S6 (R1) guidelines for non-clinical safety evaluation of biological products. The surrogate antibody-drug conjugate (ADC) used the same linker-drug as polatuzumab vedotin, namely the microtubule inhibitor MMAE, via a cleavable dipeptide valine-citrulline (vc) linker, and the average DAR value of the vc-MMAE molecule binding to anti-CD79b monoclonal antibody (mAb) was similar, but the surrogate antibody differed from the clinical antibody in that it was a chimeric structure with a non-humanized (mouse) complementarity-determining region. When using surrogate antibodies, it is essential to adequately characterize the surrogates used for safety evaluations of ADCs (e.g., epitope binding, activity, potency, and PK).
Another example of a humanized ADC is Anetumab -ravtansine, which is a humanized anti-mesothelin antibody conjugated to DM4 (a maytansinoid microtubule inhibitor), displaying effective killing action against mesothelin-expressing tumor cells. Since the ADC binds only to human mesothelin, it cannot be studied for target-mediated toxicity in non-clinical animal models; however, ADC-related off-target toxicity was assessed in rats and cynomolgus monkeys. It is believed that the toxicity observed in humans may be related to the physiological expression of mesothelin in healthy tissues, which limits the maximum tolerated dose in clinical trials to below the predictions made in non-clinical studies.
The expression of target antigens in normal tissues is a major safety concern for ADC therapy. Ideally, the expression of target antigens on normal cells should be very low; however, as seen in the two examples above, antibodies are not practically so. Other potential toxicities related to lack of specificity for ADC target sites include NaPi2b, a sodium-dependent phosphate transporter, which is expressed in several tumor types but is also detectable in normal tissues, playing a role in inorganic phosphate homeostasis. An ADC (anti-NaPi2b-vc-MMAE) was confirmed to have specific binding affinity in normal human and cynomolgus monkey tissues when conjugated with humanized IgG1 anti-NaPi2b monoclonal antibody via the vc peptide linker. However, despite high expression in normal monkey lung, the safety of this cross-reactivity can be deemed acceptable, exhibiting dose-limiting toxicity unrelated to normal tissue expression. In normal rats, non-binding species, and monkeys, the toxicological effects were consistent with the pharmacology of MMAE.
General Toxicology Design Principles: Generally, monoclonal antibodies/naked antibodies, Linker/Linker-toxin often do not require separate control groups, as the monoclonal antibody molecule is primarily one of the major factors affecting or determining systemic exposure; dose design needs to comprehensively consider the pharmacodynamic dose of ADCs, the characteristics of the antibody molecule, and the toxic characteristics of the small molecule toxin.
Focus on Payload-related Toxicity: Although targeted release of small molecular compounds achieves drug enrichment in target tissues and enhances the therapeutic window, the main toxic reaction characteristics of ADCs still resemble those of the linked small molecular compounds. Most ADCs display reduced toxicity compared to direct administration of small molecular compounds, but some ADCs may increase tissue toxicity reactions due to antibody-target binding or off-target binding. Although ADCs enhance the targeted release of small molecular compounds, a portion of the small molecular compounds may be released prematurely; some free small molecular compounds can also rapidly diffuse or transport from target cells to surrounding tissues and systemic circulation, or the apoptosis or damage of target cells may lead to the entry of free small molecular compounds into systemic circulation. The antibody portion in ADCs may also bind to surface receptors or antigen epitopes on other non-target tissue organ cells, for example, through Fc receptor-mediated phagocytosis, where the drug may produce toxic effects on corresponding tissue cells after metabolic decomposition. Therefore, the toxic effects of ADCs are closely related to the characteristics of the antibody, small molecular compounds, and linkers, and as the antibody, small molecular compounds, and linkers change, their toxicity reaction characteristics will also vary. In non-clinical safety studies, attention should be paid to the influence of drug composition structure and pharmacokinetic characteristics on toxic effects, and a comprehensive analysis of experimental results should be conducted.
Genotoxicity: Generally, biological macromolecules do not directly interact with DNA; ADCs and their antibody portions usually do not require genotoxicity testing. The potential genotoxicity of ADCs arises from small molecular compounds. If the payload small molecular compound is a novel compound, genotoxicity testing should be conducted; for newly generated free small molecular compounds and/or new linkers in the body, the target detection objects for relevant genotoxicity studies should be evaluated based on the physicochemical properties, cleavage characteristics, and structures of the degradation products of the ADC; if sufficient data indicate that the target detection object has genotoxicity, genotoxicity studies are not required.
Reproductive Toxicity: The small molecular compounds, linkers, and naked antibodies in ADCs may have adverse effects on reproductive organs, fertility, embryonic-fetal development, and offspring development; hence, the reproductive toxicity risk of ADCs should be considered. The research strategies, experimental designs, implementations, and evaluations for reproductive toxicity evaluations of ADCs should refer to ICH S5 guidelines, while combining the mechanism of action of ADCs and the characteristics of the population for which they are indicated, employing a specific problem analysis evaluation strategy. ADCs intended for patients with advanced tumors may refer to ICH S9 guidelines for conducting reproductive toxicity studies.
For biological products, reproductive toxicity is typically evaluated in pharmacologically relevant animal species. If both rodents and rabbits are the target binding-related animal species, two animal species should be used for embryonic-fetal development toxicity tests unless embryonic lethality or teratogenicity has been confirmed in one species. If the target binding animal species are non-human primates or there are no relevant animal species, it is usually first considered to use rats or rabbits for reproductive toxicity tests, examining the reproductive toxicity of free small molecular compounds. If the study results confirm positive findings, there is no need to further investigate reproductive toxicity in target binding-related animal species; otherwise, it may be necessary to consider using non-human primates or transgenic animals or surrogate molecules for testing to identify antigen-mediated reproductive toxicity. If the antibody or small molecular compound in the ADC has literature data clearly indicating potential reproductive toxicity risks, reproductive toxicity studies are not required, and relevant risk controls can be based on literature information.
Carcinogenicity: The necessity of carcinogenicity studies can be considered based on the characteristics of the ADC, following relevant guidelines such as ICH S1, ICH S6, and ICH S9.
Immunogenicity/Immunotoxicity: After entering the biological system, ADCs may induce immunogenicity, producing anti-drug antibodies. Evaluating the immunogenicity of ADCs helps analyze the pharmacokinetics, efficacy, and safety outcomes of the drug; therefore, typically, anti-drug antibody detection should accompany non-clinical safety studies of ADCs. Anti-drug antibodies may arise from the antibody portion and linker part of the ADC, and whether further investigation of antigen epitopes is needed should be considered based on immunogenicity risk. The development, validation, and analysis detection strategies for immunogenicity analysis methods for ADCs should follow the “Technical Guidelines for Drug Immunogenicity Research.” Relevant guidelines such as ICH S6, ICH S8, and ICH S9 should be referenced, and reasonable designs for immunotoxicity detection indicators should be made based on the type of ADC, mechanism of action, and other factors. In addition to conventional immunotoxicity studies, further additional immunotoxicity studies should be considered when necessary.
Phototoxicity: Before phase I clinical trials, the potential phototoxicity should be preliminarily assessed based on the photochemical properties of small molecular compounds (including linkers) and their pharmacological/chemical categories. If these data assessments indicate potential risks, appropriate protective measures should be taken for clinical trial subjects. If the phototoxicity risk cannot be sufficiently evaluated based on non-clinical data or clinical experience, a phototoxicity assessment following the principles outlined in ICH S10 should be provided before large-scale clinical trials (phase III).
Tissue Cross-Reactivity: Tissue cross-reactivity studies are conducted using immunohistochemical techniques to determine the binding characteristics of antibodies to antigen epitopes within tissues. Tissue cross-reactivity studies can provide useful supplementary information for understanding target distribution and also provide information on potential unexpected bindings. Studies using human tissues for tissue cross-reactivity are an organic component of the series of safety evaluations supporting the initial clinical administration of antibody drugs. When there are no target binding-related animal species, tissue cross-reactivity information is particularly important for predicting human toxicity risks. For ADCs intended for advanced tumor indications, toxicological effects may be evaluated using relevant animal species, and when there are no special concerns, tissue cross-reactivity studies are not mandatory.
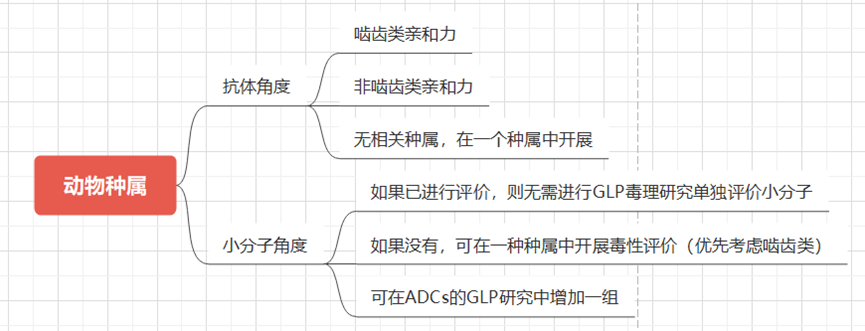
Figure 4 Animal Species Selection Strategy
Conclusion
Currently, the non-clinical pharmacology and toxicology research of ADCs has relevant technical guidelines issued by ICH, FDA, NMPA, etc. In the research, ADCs can also refer to ICHS6 and ICHS9 guidelines, while designing scientifically reasonable non-clinical pharmacology and toxicology studies based on the characteristics of the drugs, maximizing the extrapolation of non-clinical animal-level pharmacodynamic effects and toxic reactions to humans, predicting the efficacy and safety of ADCs in clinical applications, thereby improving the success rate of clinical trials and reducing development risks. New drug developers can communicate with review agencies regarding preliminary research results and existing issues to obtain scientifically standardized non-clinical research support information.
References:
Technical Guidelines for Non-Clinical Research of Antibody-Drug Conjugates. (NMPA, September 25, 2023)
Fisher JE Jr. Considerations for the Nonclinical Safety Evaluation of Antibody-Drug Conjugates. Antibodies (Basel). 2021 Apr 19;10(2):15. doi: 10.3390/antib10020015. PMID: 33921632; PMCID: PMC8167597.
Yan Liping, Wang Haixue, Wang Qingli. Key Considerations for Non-Clinical Pharmacology and Toxicology Research of Anti-Tumor Antibody-Drug Conjugates [J]. Chinese New Drug Journal, 2017, 26(16):1894-1899.
-
Some materials referenced the ADC non-clinical evaluation strategy lecture (Tang Napin. 2024-01-24)
Author: Sailing on a Ship
Disclaimer
The rules for pushing WeChat public accounts have changed again. If you do not click on “Looking” or have not set it as “Starred,” we may dissipate in the vast sea of articles~ Click here, and don’t miss the latest news from Yao Du! 👇👇👇