
To deliver chemotherapy drugs more effectively, small molecule drug conjugates (SMDC), antibody-drug conjugates (ADC), and degrader-antibody conjugates (DAC) have been explored and developed, providing selective delivery while improving the therapeutic index. What are their similarities and differences? What are their respective advantages? What is the current state of research? What does the future hold? This article analyzes these questions one by one.
Definition:
SMDC is composed of a small molecule drug (usually a highly active chemotherapy agent) and a targeted ligand (usually a small molecule), connected through conjugation technology. This conjugate can specifically deliver the drug to disease-related cells, such as cancer cells, thereby enhancing efficacy and reducing toxicity to normal tissues.
ADC is a complex composed of an antibody and a drug (usually a cytotoxic agent). The antibody portion specifically targets certain cancer cell surface antigens, while the drug portion is responsible for killing these bound cancer cells. The design of ADCs aims to directly deliver drugs to cancer cells, thereby minimizing impact on normal cells.
DAC is an emerging drug design that combines the targeting ability of antibodies with small molecules used to induce protein degradation. The antibody part targets specific proteins, while the degrader part prompts these proteins to be recognized and eliminated by the cell’s degradation system. The goal of DAC is to treat diseases by targeting and degrading pathological proteins, such as degrading key proteins on the surface of tumor cells in cancer treatment.
01
Common Features of SMDC, ADC, and DAC
SMDC, ADC, and DAC all utilize advanced structural optimization technologies, combining effective drug components with targeting strategies that enhance drug specificity, providing more precise and effective methods for disease treatment.
a. Targeting: SMDC, ADC, and DAC all possess targeting capabilities, using specific molecules (antibodies or small molecules) to accurately target disease-related cells or proteins. This targeting reduces the impact on normal cells, enhancing the specificity and effectiveness of treatment.
b. Composition of Conjugates: All are composite drugs formed by the combination of two or more different components. Whether SMDC, ADC, or DAC, they all contain active components for treatment (such as chemotherapy drugs or degraders) and guiding molecules (such as antibodies or small molecules) for directing the drug to specific targets.
c. Primarily Used for Cancer Treatment: SMDC, ADC, and DAC are mainly used for cancer treatment. They reduce tumor growth or promote the death of tumor cells by targeting cancer cells or specific proteins associated with cancer progression.
d. Innovative Biotech Products: SMDC, ADC, and DAC are all products of recent biotechnological developments, representing advanced technologies and innovative ideas in drug discovery and cancer treatment.
02
All three drug conjugates share common advantages in enhancing drug targeting, reducing side effects, and treating difficult-to-treat diseases, while each conjugate has its unique advantages that play significant roles in different therapeutic scenarios.
Common Advantages:
a. Targeted Therapy: All three conjugates provide more precise targeted therapy options, effectively locating diseased cells and reducing damage to normal cells compared to traditional therapies.
b. Reduced Side Effects: Due to their targeting capabilities, these conjugates can enhance drug efficacy while lowering toxicity and side effects.
c. Treatment of Difficult-to-Treat Diseases: They offer new avenues for treating certain difficult-to-treat diseases (such as some types of cancer).
Unique Advantages of SMDC:
a. Small Molecule Characteristics: The small molecule characteristics of SMDC allow it to penetrate cells more easily, targeting internal sites for treatment.
b. Cost Advantage: Compared to ADC and DAC, SMDC generally has lower production costs and is easier to produce on a large scale.
Unique Advantages of ADC:
a. High Specificity: The high specificity of the antibody component allows ADCs to precisely target specific cell surface antigens.
b. High Drug Carrying Capacity: ADCs can carry higher doses of drugs, enhancing therapeutic effects.
c. Wide Applicability: ADCs have a broad application in cancer treatment, with many ADC drugs already on the market or in development.
Unique Advantages of DAC:
a. Novel Mechanism: DAC works by degrading target proteins, representing a new therapeutic strategy distinct from traditional drug mechanisms.
b. Avoidance of Drug Resistance: Treating diseases by degrading target proteins may help avoid certain drug resistance issues.
03
These three drugs exhibit significant differences in carrier types, mechanisms of action, drug release methods, and clinical applications.
3.1 Carrier Types
SMDC: Uses small molecule compounds as carriers, which can more easily penetrate cell membranes and tissue barriers.
ADC: Uses antibodies as carriers, which are large molecular biopharmaceuticals that can specifically recognize and bind to certain antigens.
DAC: Also uses antibodies as carriers, but its purpose is to deliver attached degraders to specific proteins to trigger their degradation.
3.2 Mechanisms of Action
SMDC: Delivers drugs into cells through the high permeability of small molecules, typically targeting intracellular sites.
ADC: Delivers chemotherapy drugs directly to cancer cells through the targeting of antibodies, which recognize and bind to specific antigens on cancer cell surfaces, leading to internalization and drug release to kill cancer cells.
DAC: Recognizes and binds to specific cell surface proteins via antibodies, triggering intracellular protein degradation mechanisms through conjugated degraders, resulting in the degradation of target proteins.
3.3 Drug Release and Activity Mechanisms
SMDC: The release of drugs typically relies on the intracellular penetration of small molecule carriers, which can enhance drug distribution and permeability in the body, allowing drugs to reach targets more effectively.
ADC: Drug release depends on the binding and internalization of antibodies with cancer cells, delivering the drug directly to targeted cancer cells, where the drug is released to kill the cells.
DAC: The active mechanism is based on the degradation of target proteins induced by degraders.
3.4 Clinical Applications and Treatment Goals
SMDC: Suitable for cases requiring drugs to penetrate deep into cells or tissues, such as certain types of cancer.
ADC: Primarily used for cancer treatment, particularly in scenarios requiring precise targeting of tumor cells.
DAC: Mainly used to treat cancer or other diseases by degrading key proteins.
04
4.1 R&D Challenges for SMDC:
Targeting and Specificity: One of the challenges for SMDC is ensuring that the drug can accurately target and act on specific pathological cells rather than normal cells.
Drug Release Mechanism: Developing effective drug release mechanisms to ensure that the drug is released at the appropriate rate and amount within target cells is also a significant challenge.
Toxicity and Side Effects: There is a need to balance the drug’s efficacy with the toxicity risk to normal tissues, minimizing side effects.
Synthesis and Production: The synthesis of SMDC is complex and costly, requiring optimization of production processes to improve efficiency and reduce costs.
4.2 R&D Challenges for ADC:
Structural Optimization: The differential expression of tumor-associated antigens (TAA) between tumor cells and normal tissues is key to reducing “on-target, off-tumor” toxicity. Thus, seeking more ideal targets is a common goal for scientists in future R&D. The “bystander effect” is a characteristic that successful ADCs generally need to possess, and balancing target efficacy with bystander effects is also a major difficulty. Existing approved ADCs primarily focus on two major classes of payloads, and more effective payload development is ongoing. Meanwhile, the optimal drug-to-antibody ratio (DAR) has not yet been established. Currently, the timing of administration and half-life do not match, and further optimization of drug exposure-response relationships remains to be resolved.
Clinical Applications: Unacceptable toxicity remains a major barrier to developing new drugs. Improving the prediction of severe adverse events will be another method to reduce premature discontinuation of drugs, and managing the toxicity of new drugs requires long-term effort. As the clinical use of ADCs increases, their cost-effectiveness will also be scrutinized. The competitive landscape of ADCs relative to other antigen-specific immunotherapy regimens is still unknown. Additionally, finding new biomarkers to guide the clinical application of ADCs is a key challenge. Similar to other anti-tumor drugs, most patients who receive ADC treatment in advanced stages and show initial remission will eventually experience tumor progression; however, compared to other anti-tumor drugs, the mechanisms of ADC resistance remain unclear and may be more complex, which is still an unavoidable issue.
4.3 R&D Challenges for DAC:
Selection of Target Proteins: Choosing appropriate target proteins is critical, ensuring that the target protein plays a significant role in the disease.
Design and Stability of Conjugates: Designing effective and stable conjugates that allow degraders to accurately target and promote the degradation of target proteins. Since the chimeric degraders may be weaker than cytotoxic ADC payloads, higher loadings may be needed to achieve similar effects (i.e., DAR greater than 4). Due to their chimeric nature, DAC PROTAC effective payloads are typically larger or more lipophilic than ADC’s cytotoxic molecules (especially cell-permeable molecules). When PROTAC is attached to antibodies, these differences can amplify aggregation and pharmacokinetic issues, potentially requiring new linker designs and conjugation methods rather than methods used in the ADC field.
Intracellular Degradation Mechanisms: Understanding and utilizing intracellular degradation mechanisms are necessary to ensure the effective operation of the drug.
Safety and Side Effects: Considering that DAC may affect the balance of multiple proteins within cells, safety and potential side effects are important considerations.
Other challenges that may need to be addressed in DAC design include: (1) good stability of DAC effective payloads in lysosomal environments; (2) the ability of effective payloads to escape lysosomal compartments; (3) the tendency of effective payloads to produce bystander effects. The latter two characteristics may be influenced by the cell permeability of the degrader itself.
Overall, these three drug conjugates need to comprehensively consider targeting, drug release, stability, safety, and production efficiency throughout the R&D process. With advancements in technology and deeper research, these challenges are expected to be gradually overcome.
05
Real Gaps in Clinical R&D
As of now, SMDC, ADC, and DAC have achieved some specific results in clinical research, but they are at different stages of development, with gaps in drugs under research or on the market.
5.1 SMDC at the Highest Stage in Clinical Phase I
SMDC research is relatively new, but it has already shown potential in some cancer treatment areas. Certain SMDC products have entered clinical trial stages, mainly focusing on the treatment of specific types of solid tumors and hematological cancers, with few specific drug examples, and most are still in early clinical or Phase I stages.
Clinical Phase I SMDC Drugs:
VIP236: VIP236 is developed by Vincerx Pharma and is considered a pioneering SMDC drug. VIP236 has a unique design aimed at effectively treating patients with aggressive and metastatic cancers. It targets activated αvβ3 integrin, specifically localizing to tumor sites and efficiently cleaving through neutrophil elastase (NE) within the tumor microenvironment. VIP236 has shown significant anti-tumor activity in preclinical studies using patient-derived mouse models across various tumor types. Additionally, VIP236 has demonstrated the ability to penetrate the blood-brain barrier, reducing brain metastases. Currently, VIP236 is undergoing Phase I human clinical dose escalation trials, primarily targeting patients with advanced solid tumors. Early safety assessments and indications of clinical activity for this drug show optimistic results. Vincerx Pharma expects to release preliminary clinical trial data in early 2024.
Enitociclib: Another drug from Vincerx Pharma, Enitociclib, is also in Phase I clinical trials. It is a highly selective CDK9 inhibitor used in combination with Venetoclax and Prednisone to treat lymphoma. This drug has already shown evidence of therapeutic effects for patients with hematological malignancies and solid tumors in early clinical studies.
No SMDC drugs in Clinical Phase II or III, and no SMDC has been officially marketed yet.
5.2 ADC Widely Applied in Tumors
ADC is the most widely clinically applied among these three drugs, with multiple ADCs approved for treating different types of cancer.
Clinical Major Targets of ADC Drugs:
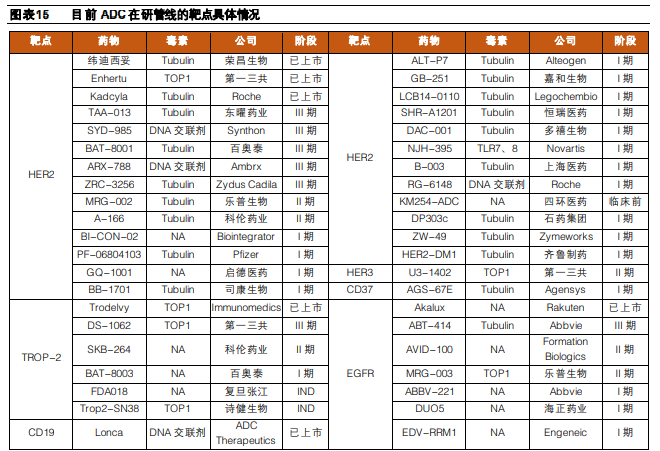
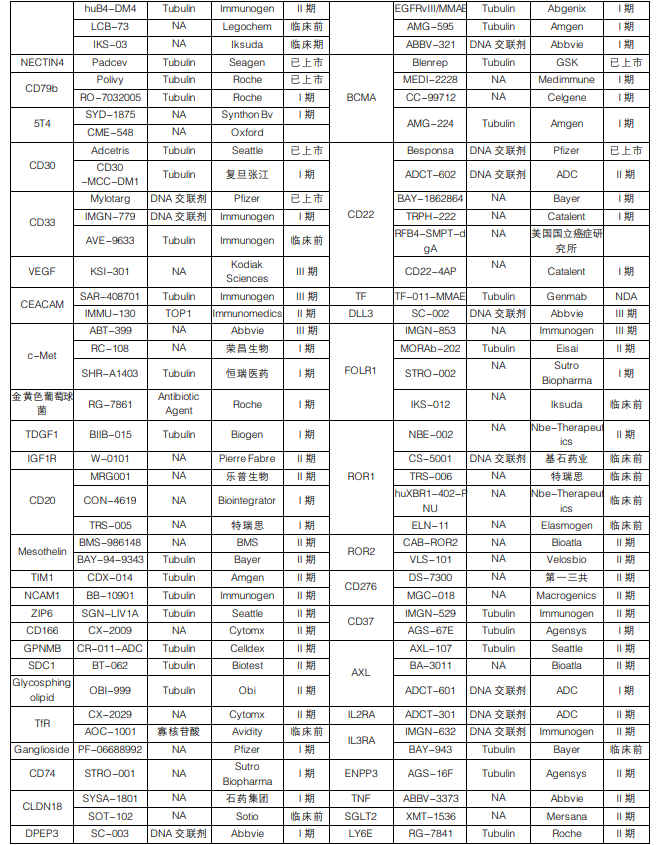
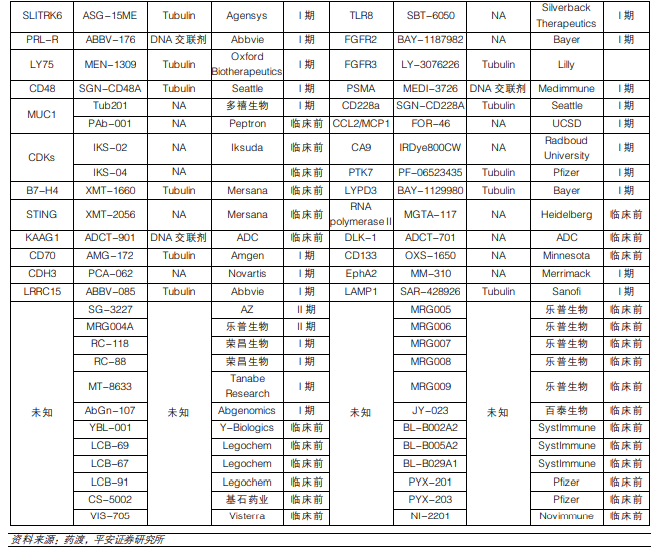 Figure Caption: ADC Drugs in Clinical Phases (Data Source: Ping An Securities)
Figure Caption: ADC Drugs in Clinical Phases (Data Source: Ping An Securities)
Globally Approved ADC Drugs:
As of the end of 2023, a total of 15 ADC drugs have been approved by the FDA worldwide. Among them, the HER2 target development pipeline is the most abundant, with cytotoxic agents mainly using MMAE-type compounds. Since the approval of Polivy in 2019, a new era of ADC innovation has officially begun, with 10 ADC drugs approved in recent years.
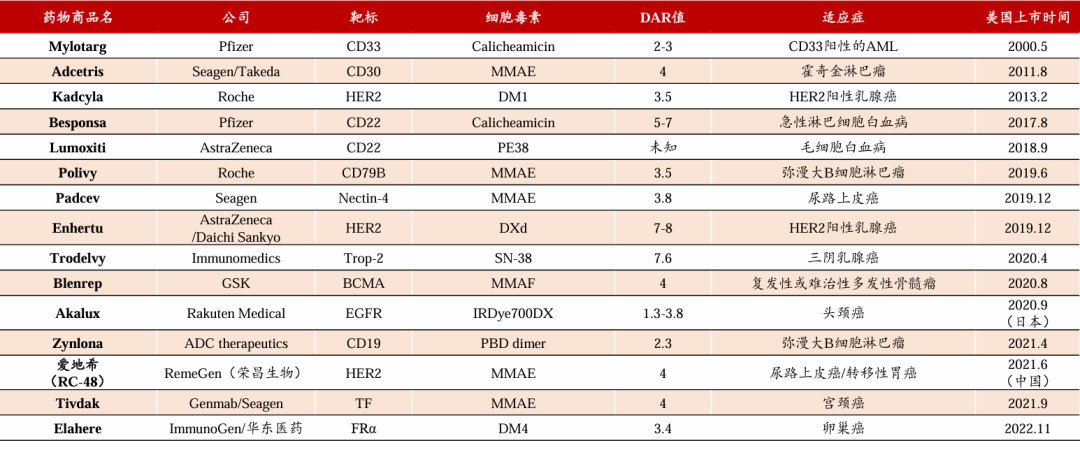 Figure Caption: Global ADC Drug Approval Status (Data Source: Guolian Securities)
Figure Caption: Global ADC Drug Approval Status (Data Source: Guolian Securities)
In China, the time to market for ADC drugs is relatively short. As of December 2023, a total of 7 ADC drugs have been approved by the NMPA. In February 2023, the blockbuster HER2 ADC drug, trastuzumab deruxtecan, developed by multinational giants Daiichi Sankyo/AstraZeneca, has been approved by the NMPA. Additionally, currently, only one HER2 ADC drug (vidisertib) is from domestic company Rongchang Biologics.
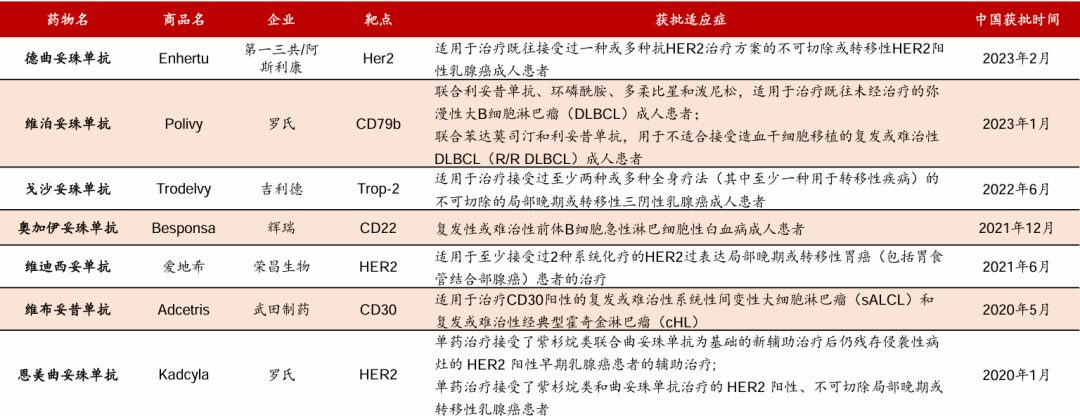 Figure Caption: Domestic ADC Drug Approval Status (Data Source: Guolian Securities)
Figure Caption: Domestic ADC Drug Approval Status (Data Source: Guolian Securities)
5.3 DAC Still in Early Clinical Stages
As an emerging therapy, DAC is still in the early stages of research and development. DAC research focuses on using antibodies to guide degraders to specific disease-related proteins and trigger their degradation to treat cancer or other diseases. Some drugs are undergoing early-stage clinical trials.
Clinical Phase DAC Drugs:
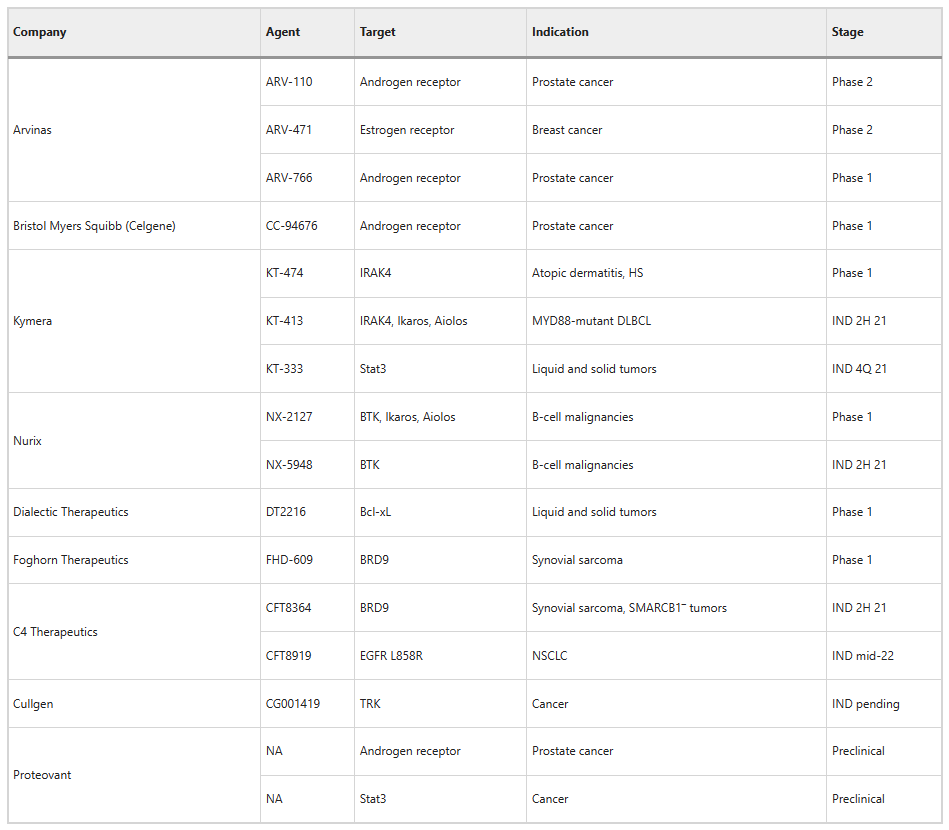 Figure Caption: Clinical Phase DAC Drugs (Nature Biotechnology, 40, 12–16, 2022)
Figure Caption: Clinical Phase DAC Drugs (Nature Biotechnology, 40, 12–16, 2022)
Summary
SMDC, ADC, and DAC are like a network of conjugates, with a very bright future in the pharmaceutical field. They show tremendous potential in treating various diseases, especially cancers, while each has its unique advantages. With the continuous advancement of synthetic technologies, conjugation technologies, targeting technologies, and structural optimization technologies, their efficacy and safety will be further improved, and their development and application will become more widespread and precise. Among them, ADC has already occupied an important position in cancer treatment, with the market size expected to continue to grow. The uniqueness and novelty of DAC’s therapeutic mechanism give it the potential to treat a variety of protein-related diseases, including those that traditional drugs find difficult to treat, offering new treatment options for difficult-to-treat diseases.
These three types of drug conjugates will play important roles in the future pharmaceutical field, especially in the treatment of cancer and other difficult-to-treat diseases. With continuous advancements in science and technology and deeper clinical research, their application scope and efficacy expectations will further expand.
Scan the QR code to add the editor of the Bioproduct Circle. Eligible individuals can join the
Bioproduct WeChat group!
Please specify: Name + Research Direction!


The articles reprinted by this public account are for the purpose of conveying more information, and the sources and authors are clearly indicated. If any media or individual does not wish to be reprinted, please contact us ([email protected]), and we will immediately take action to delete it. All articles only represent the author’s views and do not represent the position of this site.