In the fields of biomedical research and clinical applications, real-time monitoring of biochemical indicators within the body is crucial. Whether it is the dynamic monitoring of blood glucose levels in diabetic patients or tracking changes in neurotransmitters in patients with neurodegenerative diseases, biochemical sensors play a key role. However, for a long time, sensors have faced numerous challenges in the in vivo environment. The complex physiological environment within the body, such as immune responses, protein adsorption, and changes in tissue fluid composition, can lead to decreased sensor performance and insufficient stability, making it difficult to achieve long-term and accurate detection. This has become a bottleneck limiting the further development and widespread application of biochemical sensors.

Interdisciplinary Innovation: The Birth of New Biochemical Sensors
Recently, a research achievement on “continuous and stable biochemical sensors in vivo” has brought new hope for solving the aforementioned problems. This research achieved breakthroughs through interdisciplinary innovative design in the intersection of materials science, nanotechnology, and biomedical engineering, successfully developing a biochemical sensor that can maintain stable operation for a long time within the body.
Bionic Interface Design: Counteracting the Complex In Vivo Environment
The researchers first focused on the interface design of the sensor. Traditional sensors, once implanted in the body, trigger immune responses that lead to the formation of fibrous encapsulation, affecting sensor performance. To address this issue, the new sensor employs bionic coating technology. Its surface is modified with a polyethylene glycol (PEG) – peptide composite coating, where polyethylene glycol reduces non-specific protein adsorption through steric hindrance effects, and the peptide sequence mimics extracellular matrix components, reducing immune cell recognition and adhesion to the sensor, thereby alleviating immune responses. Additionally, the sensor introduces a pH-responsive hydrogel layer, which expands to form a physical barrier when the local microenvironment experiences a decrease in pH due to inflammatory responses, further isolating immune cells from the sensor interface and effectively protecting the sensor’s detection function.
Signal Processing Innovation: Ensuring Detection Accuracy
In terms of signal processing, the sensor has also undergone innovative improvements. The sensor electrodes are loaded with platinum nanoenzymes, which mimic peroxidase activity and can efficiently convert changes in the concentration of target molecules (such as hydrogen peroxide) into detectable current signals, significantly enhancing detection sensitivity to the nanomolar level. Meanwhile, the sensor integrates an Ag/AgCl solid-state reference electrode, which dynamically corrects the potential drift of the working electrode by real-time monitoring of electrolyte ion strength changes, ensuring the long-term accuracy of detection results with an error control within 3%.
Material and Structural Optimization: Enhancing Biocompatibility
In addition to interface and signal processing technologies, the choice of materials and structural design of the sensor is equally important. The new sensor uses a polyimide (PI) – PDMS composite film as the substrate. This flexible substrate material has an elastic modulus that matches soft tissues, reducing tissue damage caused by mechanical stress after implantation. Furthermore, the sensor is surrounded by a polylactic acid – glycolic acid copolymer (PLGA) microsphere array, which provides mechanical support in the early stages of implantation. Over time, the microspheres gradually degrade and release anti-inflammatory factors, achieving the dual goal of “short-term stability – long-term compatibility,” ensuring the safety and stability of the sensor in vivo.
Animal Experiment Validation: Strong Evidence of Excellent Performance
To validate the performance of the new biochemical sensor, the research team conducted extensive animal experiments. In rat and pig models, the sensor was implanted and observed for up to 12 weeks. The experimental results were encouraging: the signal fluctuation for glucose detection was less than 5%, significantly outperforming traditional carbon electrode sensors; after injecting substances that could interfere with detection, such as bovine serum albumin or lipopolysaccharides that trigger inflammatory responses, the signal drift was <8%, while the control group sensors without special design drifted over 40%. Histological sections showed only a small amount of fibroblast infiltration around the new sensor, with no significant granuloma formation, and the inflammation score was reduced by 70% compared to traditional titanium alloy electrodes, fully demonstrating its good biocompatibility and stability.
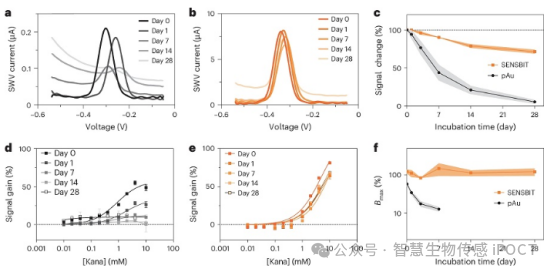
Figure 1: Performance of SENSBIT after one month of incubation in human serum in vitro
Clinical Application Prospects and Future Challenges
This research achievement has significant clinical translational implications. For diabetic patients, a sensor capable of real-time, accurate, and long-term monitoring of blood glucose can help achieve more effective blood glucose management and reduce the risk of complications. In the study of neurodegenerative diseases, it can track the dynamic changes of neurotransmitters in the brain in real-time, providing key data for revealing disease mechanisms and assessing treatment effects. Additionally, during cancer treatment, monitoring biochemical indicators in the tumor microenvironment is expected to enable the development of personalized treatment plans.
Of course, to truly apply this technology in clinical settings, there are still many challenges to overcome. In the future, it will be necessary to further develop multi-parameter integrated sensors to achieve simultaneous monitoring of multiple biochemical indicators; combine flexible electronic technology to solve wireless power supply and data transmission issues, enabling wireless implantation and real-time remote data analysis; and conduct comprehensive assessments of long-term implantation safety and effectiveness in more large animal models and clinical trials, optimizing material degradation kinetics, etc.
The successful development of the new biochemical sensor is an important breakthrough in the field of biomedical engineering. It provides a new approach for achieving continuous and stable monitoring of biochemical indicators in vivo. Although there are still many difficulties to overcome ahead, the immense potential demonstrated by this technology fills us with anticipation for the future of precision medicine. With continuous improvement and innovation in technology, we believe that in the near future, long-lasting and stable biochemical sensors will be widely used in clinical settings, providing better medical services and health management solutions for patients.