Immunotherapy shows great promise in cancer treatment. However, the highly immunosuppressive nature of solid tumors limits its effectiveness.
On October 15, 2024, the research paper titled “Zinc-Nickel Bimetallic Hydroxide Nanosheets Activate the Paraptosis–Pyroptosis Positive Feedback Cycle for Enhanced Tumor Immunotherapy” was published online in ACS Nano by Cheng Liang from Soochow University and Hou Guanghui from Soochow University in collaboration with the University of Macau. This study developed bioactive zinc-nickel hydroxide (ZnNi(OH)4) nanosheets (NSs) that initiate a death-pyroptosis positive feedback loop through a synergistic ionic effect, alleviating the immune suppression of solid tumors and enhancing the efficacy of immunotherapy.
The acid-responsive ZnNi(OH)4 NSs release Ni2+ and Zn2+ in the weakly acidic tumor microenvironment. The released Ni2+ alleviates pyroptosis suppression by inducing paraptosis and inhibiting autophagic flux. Meanwhile, Ni2+ triggers the release of endogenous Zn2+ through a coordination competition mechanism, further amplifying the pyroptosis mediated by zinc overload. Interestingly, pyroptosis-related oxidative stress and endoplasmic reticulum stress further promote Ni2+-mediated apoptosis, forming a positive feedback loop between pyroptosis and apoptosis, effectively killing tumor cells while stimulating a strong inflammatory response, enhancing anti-tumor immune response and immunotherapy efficacy. Overall, this study proposes a metal ion-based apoptosis-pyroptosis induction strategy and demonstrates the effectiveness of the apoptosis-pyroptosis positive feedback loop in enhancing immunotherapy.
 Immunotherapy has brought a new revolution in cancer treatment. Unfortunately, insufficient immune responses and a highly immunosuppressive microenvironment within solid tumors severely hinder the effectiveness of immunotherapy, making the development of new strategies to enhance immunotherapy efficacy a current research focus. Precisely regulating inflammatory cell death in tumor cells (such as necroptosis, ferroptosis, cuproptosis, and pyroptosis) is an effective method to reshape the tumor immune microenvironment and enhance the immunotherapy response. Among various forms of inflammatory cell death, pyroptosis has garnered widespread attention from researchers due to its ability to enhance immunogenicity. In contrast to apoptosis, pyroptosis is a unique form of cell death characterized by cell swelling, membrane rupture, chromatin degradation, and the release of pro-inflammatory cellular contents; unlike apoptotic cells, pyroptotic cells are rapidly engulfed, thus lacking an inflammatory response.
During pyroptosis, inflammasomes trigger the activation of caspase-1 and the release of pro-inflammatory cytokines, while caspase-1 cleaves gasdermin D to release its N-terminal domain (GSDMD-N), which affects cell death and the release of inflammatory contents through its pore-forming activity. Various biotechnologies such as chemotherapy, radiotherapy, photodynamic therapy, and sonodynamic therapy can effectively trigger pyroptosis, thereby activating a strong immune response. However, recent studies have shown that intrinsic survival mechanisms of cancer cells, such as autophagy, can inhibit the activation of inflammasomes and inflammatory cytokines, promoting their degradation and thereby suppressing the induced immunotherapy effect. Moreover, specific induction of pyroptosis in cancer cells still faces certain difficulties, necessitating the urgent exploration of new strategies that can effectively enhance pyroptosis and immunotherapy efficacy.
Paraptosis is a unique form of inflammatory cell death proposed in 2000, which differs from classical apoptosis. As a caspase-independent programmed cell death pathway, paraptosis does not involve the formation of apoptotic bodies or chromatin condensation and is not influenced by caspase or autophagy inhibitors. Therefore, this process leads to the release of inflammatory substances, stimulating immune cells and enhancing the immune response. Although the exact triggering mechanism of paraptosis is not fully understood, its occurrence is typically associated with mitochondrial and endoplasmic reticulum (ER) stress.
Research has shown that severe ER stress during paraptosis leads to the release of large amounts of Ca2+ from the endoplasmic reticulum calcium pool into the cytoplasm, activating many death-related protein kinases and calcium-dependent protein kinases. These kinases cause autophagic distortion, inhibit the fusion of lysosomes with autophagosomes to form autolysosomes, disrupt autophagic flux, and reduce the degradation of SQSTM1/p62, thereby decreasing the cell’s resistance to pyroptosis. In addition, ER stress activates the NLRP3 inflammasome and its downstream caspase-related pathways, amplifying the pyroptosis effect. Pyroptosis-induced oxidative and cellular stress further exacerbates organelle stress, forming a positive feedback loop involving paraptosis. Therefore, the precise combination of paraptosis and pyroptosis is a potential strategy for effectively eliminating tumor cells while triggering a robust immune response. However, the precise induction of paraptosis and pyroptosis to enhance immunotherapy efficacy still faces significant challenges.
Immunotherapy has brought a new revolution in cancer treatment. Unfortunately, insufficient immune responses and a highly immunosuppressive microenvironment within solid tumors severely hinder the effectiveness of immunotherapy, making the development of new strategies to enhance immunotherapy efficacy a current research focus. Precisely regulating inflammatory cell death in tumor cells (such as necroptosis, ferroptosis, cuproptosis, and pyroptosis) is an effective method to reshape the tumor immune microenvironment and enhance the immunotherapy response. Among various forms of inflammatory cell death, pyroptosis has garnered widespread attention from researchers due to its ability to enhance immunogenicity. In contrast to apoptosis, pyroptosis is a unique form of cell death characterized by cell swelling, membrane rupture, chromatin degradation, and the release of pro-inflammatory cellular contents; unlike apoptotic cells, pyroptotic cells are rapidly engulfed, thus lacking an inflammatory response.
During pyroptosis, inflammasomes trigger the activation of caspase-1 and the release of pro-inflammatory cytokines, while caspase-1 cleaves gasdermin D to release its N-terminal domain (GSDMD-N), which affects cell death and the release of inflammatory contents through its pore-forming activity. Various biotechnologies such as chemotherapy, radiotherapy, photodynamic therapy, and sonodynamic therapy can effectively trigger pyroptosis, thereby activating a strong immune response. However, recent studies have shown that intrinsic survival mechanisms of cancer cells, such as autophagy, can inhibit the activation of inflammasomes and inflammatory cytokines, promoting their degradation and thereby suppressing the induced immunotherapy effect. Moreover, specific induction of pyroptosis in cancer cells still faces certain difficulties, necessitating the urgent exploration of new strategies that can effectively enhance pyroptosis and immunotherapy efficacy.
Paraptosis is a unique form of inflammatory cell death proposed in 2000, which differs from classical apoptosis. As a caspase-independent programmed cell death pathway, paraptosis does not involve the formation of apoptotic bodies or chromatin condensation and is not influenced by caspase or autophagy inhibitors. Therefore, this process leads to the release of inflammatory substances, stimulating immune cells and enhancing the immune response. Although the exact triggering mechanism of paraptosis is not fully understood, its occurrence is typically associated with mitochondrial and endoplasmic reticulum (ER) stress.
Research has shown that severe ER stress during paraptosis leads to the release of large amounts of Ca2+ from the endoplasmic reticulum calcium pool into the cytoplasm, activating many death-related protein kinases and calcium-dependent protein kinases. These kinases cause autophagic distortion, inhibit the fusion of lysosomes with autophagosomes to form autolysosomes, disrupt autophagic flux, and reduce the degradation of SQSTM1/p62, thereby decreasing the cell’s resistance to pyroptosis. In addition, ER stress activates the NLRP3 inflammasome and its downstream caspase-related pathways, amplifying the pyroptosis effect. Pyroptosis-induced oxidative and cellular stress further exacerbates organelle stress, forming a positive feedback loop involving paraptosis. Therefore, the precise combination of paraptosis and pyroptosis is a potential strategy for effectively eliminating tumor cells while triggering a robust immune response. However, the precise induction of paraptosis and pyroptosis to enhance immunotherapy efficacy still faces significant challenges.
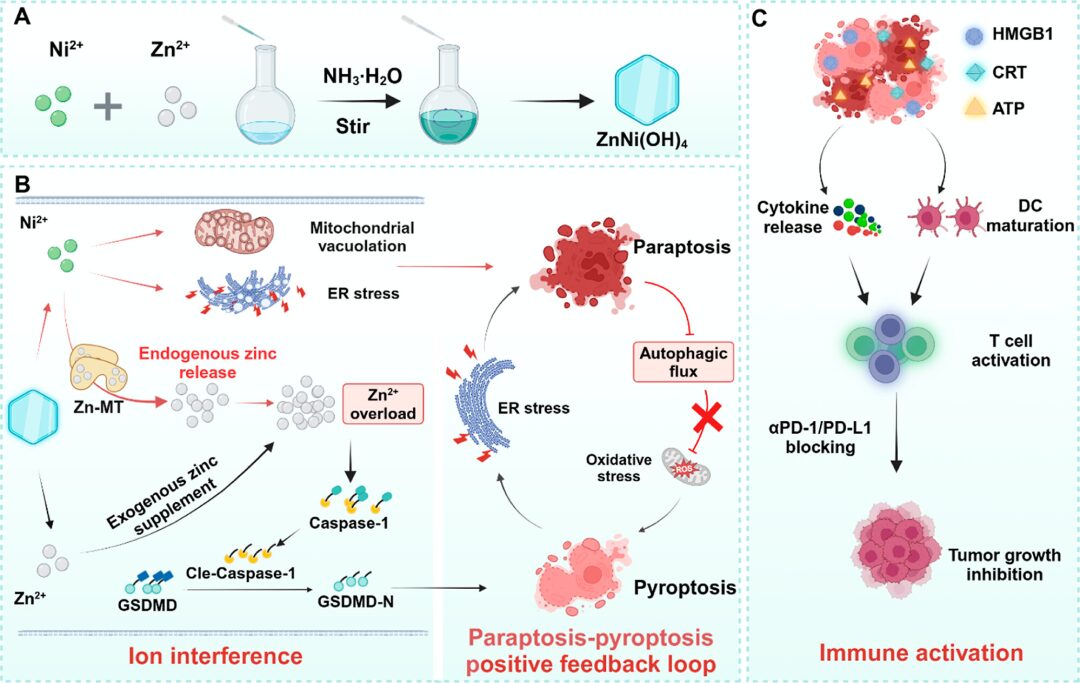 Figure 1 ZnNi(OH)4 Induces Ionic Interference to Enhance Cancer Cell Immunogenicity for Improved Immunotherapy Efficacy (Excerpt from ACS Nano)
The metal ion interference strategy can accurately regulate cell fate. Disruption of ion distribution and concentration within cells can affect the composition of proteins and enzymes, catalyze reactions, and activate signaling pathways, leading to irreversible physical damage to cells, allowing precise control over cell death, with advantages such as high efficiency and no drug resistance. Elements such as manganese (Mn), iron (Fe), copper (Cu), and calcium (Ca) can effectively activate innate or adaptive immune responses by triggering different biological effects, including activation of the STING pathway, ferroptosis, cuproptosis, and calcium overload, thereby increasing the sensitivity of solid tumors to immunotherapy. Thus, metal ion interference is an effective strategy for synergistically inducing paraptosis and pyroptosis to improve immunotherapy efficacy.
To design efficient paraptosis-pyroptosis inducers, the authors studied the potential anti-tumor effects of various metal ions and found that Ni2+ and Zn2+ induce paraptosis and pyroptosis, respectively, and their combined use can effectively initiate the paraptosis-pyroptosis positive feedback loop through synergism. Thus, this study prepared zinc-nickel hydroxide (ZnNi(OH)4) nanosheets (NSs) as ionic carriers for inducing paraptosis-pyroptosis to improve the immune microenvironment. The acid-sensitive ZnNi(OH)4 responds to the weakly acidic tumor microenvironment by releasing Ni2+ and Zn2+, where Ni2+ alleviates pyroptosis suppression by inducing ER stress and inhibiting autophagic flux, inducing the release of endogenous zinc ions through a coordination competition mechanism.
The endogenous Zn2+ released by cancer cells interacts with the exogenous Zn2+ provided by ZnNi(OH)4, leading to zinc overload in tumor cells. The low expression of metallothionein (MT) in normal tissues does not provide sufficient endogenous Zn2+ to reach the toxicity threshold of Zn2+, but initiates metal ion detoxification mediated by zinc metabolism, resulting in tumor-specific Zn2+ overload that leads to pyroptosis. Due to the effective synergistic biological effects of Ni2+ and Zn2+, ZnNi(OH)4 triggers a positive feedback loop between paraptosis and pyroptosis. This process not only effectively kills tumor cells but also stimulates a strong inflammatory response, significantly enhancing anti-tumor immune response and immunotherapy efficacy. In summary, this study proposes an effective strategy for reshaping the tumor microenvironment and discovers the potential synergistic effects of paraptosis and pyroptosis in enhancing immunotherapy efficacy.
https://pubs.acs.org/doi/10.1021/acsnano.4c10378
Figure 1 ZnNi(OH)4 Induces Ionic Interference to Enhance Cancer Cell Immunogenicity for Improved Immunotherapy Efficacy (Excerpt from ACS Nano)
The metal ion interference strategy can accurately regulate cell fate. Disruption of ion distribution and concentration within cells can affect the composition of proteins and enzymes, catalyze reactions, and activate signaling pathways, leading to irreversible physical damage to cells, allowing precise control over cell death, with advantages such as high efficiency and no drug resistance. Elements such as manganese (Mn), iron (Fe), copper (Cu), and calcium (Ca) can effectively activate innate or adaptive immune responses by triggering different biological effects, including activation of the STING pathway, ferroptosis, cuproptosis, and calcium overload, thereby increasing the sensitivity of solid tumors to immunotherapy. Thus, metal ion interference is an effective strategy for synergistically inducing paraptosis and pyroptosis to improve immunotherapy efficacy.
To design efficient paraptosis-pyroptosis inducers, the authors studied the potential anti-tumor effects of various metal ions and found that Ni2+ and Zn2+ induce paraptosis and pyroptosis, respectively, and their combined use can effectively initiate the paraptosis-pyroptosis positive feedback loop through synergism. Thus, this study prepared zinc-nickel hydroxide (ZnNi(OH)4) nanosheets (NSs) as ionic carriers for inducing paraptosis-pyroptosis to improve the immune microenvironment. The acid-sensitive ZnNi(OH)4 responds to the weakly acidic tumor microenvironment by releasing Ni2+ and Zn2+, where Ni2+ alleviates pyroptosis suppression by inducing ER stress and inhibiting autophagic flux, inducing the release of endogenous zinc ions through a coordination competition mechanism.
The endogenous Zn2+ released by cancer cells interacts with the exogenous Zn2+ provided by ZnNi(OH)4, leading to zinc overload in tumor cells. The low expression of metallothionein (MT) in normal tissues does not provide sufficient endogenous Zn2+ to reach the toxicity threshold of Zn2+, but initiates metal ion detoxification mediated by zinc metabolism, resulting in tumor-specific Zn2+ overload that leads to pyroptosis. Due to the effective synergistic biological effects of Ni2+ and Zn2+, ZnNi(OH)4 triggers a positive feedback loop between paraptosis and pyroptosis. This process not only effectively kills tumor cells but also stimulates a strong inflammatory response, significantly enhancing anti-tumor immune response and immunotherapy efficacy. In summary, this study proposes an effective strategy for reshaping the tumor microenvironment and discovers the potential synergistic effects of paraptosis and pyroptosis in enhancing immunotherapy efficacy.
https://pubs.acs.org/doi/10.1021/acsnano.4c10378

—END—
The content is original from 【iNature】.
Reprint must state the source from 【iNature】.
Add to WeChat Group
iNature gathers 40,000 life science researchers and doctors. We have formed 80 comprehensive groups (16 PI groups and 64 doctoral groups), and also established specialized groups in relevant fields (plants, immunology, cells, microbiology, gene editing, neuroscience, chemistry, physics, cardiovascular, oncology, etc.).Warm reminder: When joining the group, please leave a note (format: school + major + name; if you are a PI/professor, please indicate that you are a PI/Professor, otherwise, it will be assumed that you are a doctoral student, thank you).You can first add the editor’s WeChat ID (love_iNature), or long-press the QR code to add the editor, and then join the relevant group, serious inquiries only.


For submission, collaboration, and reprint authorization, please contact WeChat ID:13701829856 or email:[email protected]
If you find this article interesting, please click here!




