Skip to content

- In the 21st century, the rapid development of human society has led to an increasing demand for green energy. Hydrogen energy, obtained through the complete decomposition of water, is not only environmentally friendly but also holds promise in addressing the intermittency issues of new energy sources. However, the slow kinetics and high overpotential of the Oxygen Evolution Reaction (OER) limit its application.
- The introduction of Tellurium (Te) is expected to bring new breakthroughs.
- Among the chalcogen elements, Te has the lowest electronegativity and possesses many unique properties such as multivalence, strong covalency, and high conductivity, making it a promising candidate for electrocatalytic OER.
- This review introduces the properties of Te, summarizes the outstanding performance of Te-doped and its compounds in OER, and highlights the scientific mechanisms by which Te promotes the kinetics of the OER process. Finally, we look forward to the challenges and development prospects of Te in OER applications.

- 01 Importance and Challenges of the Oxygen Evolution Reaction
-
The Oxygen Evolution Reaction is one of the important half-reactions in overall water splitting, involving a four-electron transfer process, with kinetics that are limited and requiring a large overpotential.
-
The slow kinetics and high overpotential restrict the application of the Oxygen Evolution Reaction.
- 02 Introduction of Tellurium
-
Tellurium is the chalcogen with the lowest electronegativity, possessing unique properties such as multivalence and strong covalency.
-
Researchers tend to obtain higher OER activity by doping with Tellurium.
- 03 Te-Doped Catalysts
-
Te doping is an effective method to enhance catalyst efficiency.
-
Te doping can distort the lattice and create defects, thereby improving the activity of the OER catalysts.
-
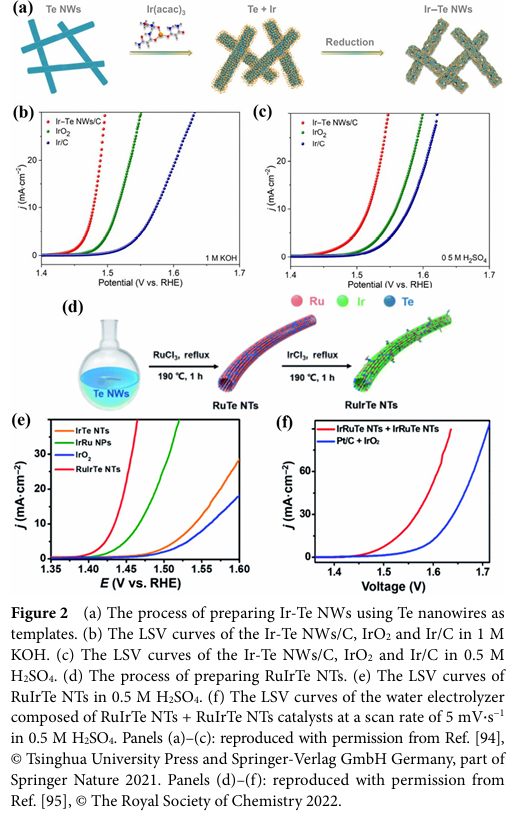
For example, Te-doped two-dimensional black phosphorus (BP), metal-organic framework (MOF) catalysts, transition metal-based LDHs, and some efficient oxides, hydroxides, and sulfides.
- 04 Te-Doped Two-Dimensional Black Phosphorus
-
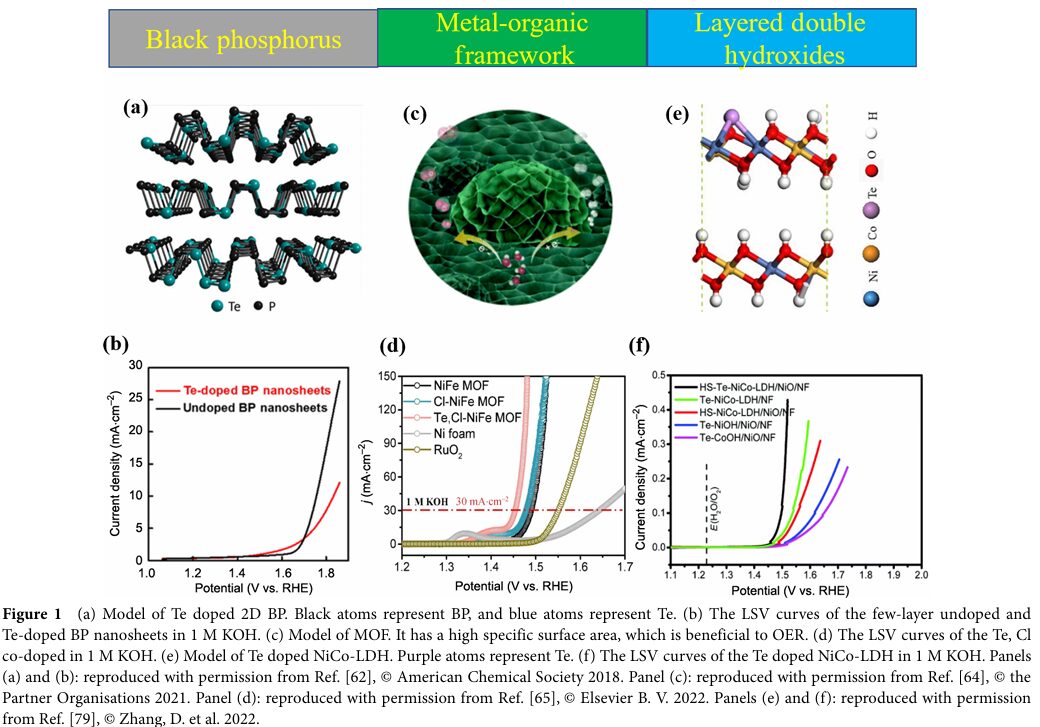
Yang, Bingchao et al. successfully doped low concentrations of Tellurium (0.1% atomic ratio) into BP using high-pressure techniques.
-
The Te-doped BP exhibited significantly improved OER efficiency, attributed to the substantial impact of Te atom introduction on the BP lattice, charge distribution, and electronic properties.
- 05 Te-Doped MOF Catalysts
-
Keemin Park et al. used chemical vapor deposition (CVD) methods to incorporate Tellurium into MOFs, achieving the growth of Te and Cl co-doped NiFe MOFs composed of two-dimensional nanosheets on nickel foam.
-
This catalyst demonstrated efficient OER in alkaline electrolytes with an overpotential of only 224 mV and stability exceeding 120 hours.

- 06 Te-Doped NiCo-LDH Catalysts
-
Using a dynamic oxygen bubble template method, Te-doped NiCo LDHs were grown on 3D porous nickel foam.
-
The doped Tellurium preferentially occupies the edge sites of transition metals and undergoes strong covalent p-d hybridization with the transition metals, forming a highly polarized local electronic structure that significantly enhances OER activity.
- 07 Influence of Te Doping on Other Catalysts
-
Te doping can also improve the activity of some common oxides, hydroxides, and sulfide compounds.
-
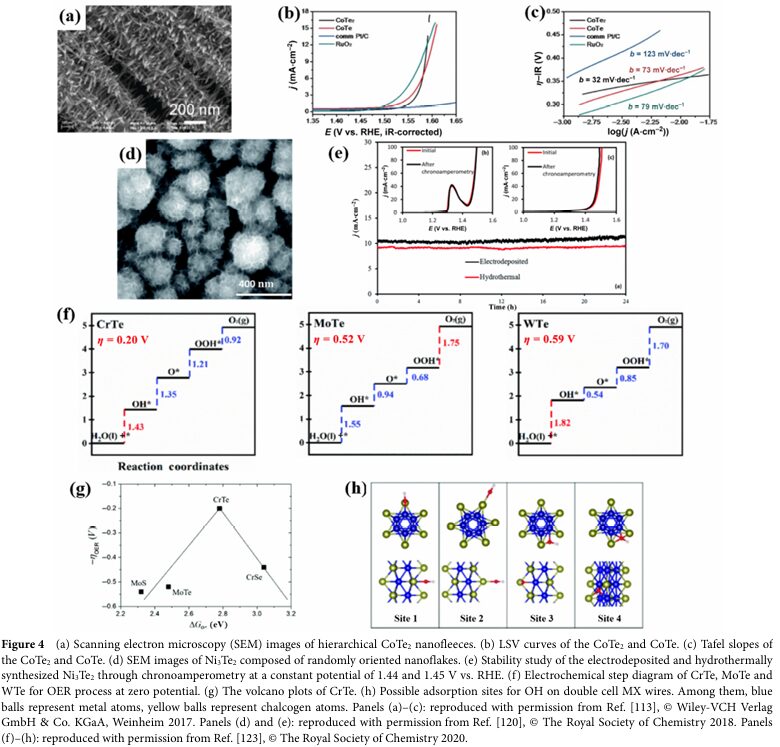
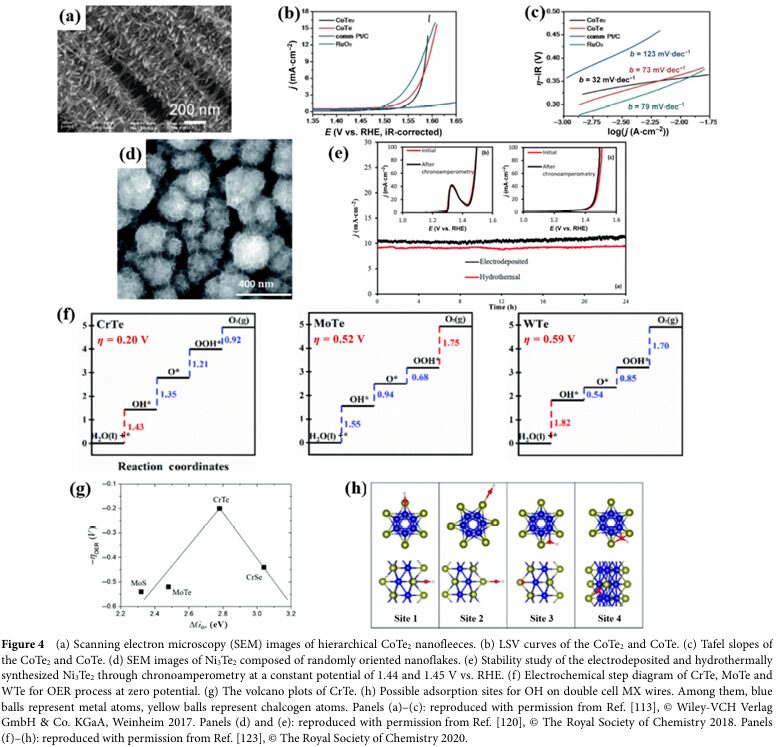
For instance, Wang, Y. et al. prepared a sandwich-structured Te-doped CoTe2xSe2(1-x) catalyst, achieving better OER performance than the undoped binary pure CoSe2 and CoTe2 species by optimizing the Te doping amount.
- 08 Mechanism of Te’s Action
-
Te doping can reduce the binding strength of intermediates *O and optimize the adsorption energy of intermediates, thereby enhancing the activity of the catalyst.
-
In some cases, Tellurium can also stabilize the catalyst, preventing its transition from the high-activity α phase to the low-activity β phase under alkaline conditions.
-
-
-
This article provides a detailed introduction to the Te-mediated electrochemical oxygen evolution reaction, optimizing the OER performance of a series of catalysts through Te doping. These catalysts include two-dimensional black phosphorus, metal-organic framework catalysts, transition metal-based LDHs, etc., all showing significantly improved OER efficiency and stability.
-
The mechanism of Te doping includes reducing the binding strength of intermediates, optimizing adsorption energy, and stabilizing catalyst structures. These findings provide new ideas and methods for the study of electrochemical oxygen evolution reactions.
This article is provided by Hydrogen Energy Research Assistant.
https://doi.org/10.26599/NRE.2022.9120029
▲ Programmable Dual-Module High-Precision Membrane Electrode Coating Machine