Globally, chronic kidney disease (CKD) is becoming an increasingly severe health threat—statistics show that it affects about 10% of the population and ranks among the top ten causes of death in low- and middle-income countries. When the condition progresses to end-stage renal disease (ESRD), patients often rely on dialysis or kidney transplantation to sustain life, but the former is only a temporary solution, while the latter faces the dual challenges of donor shortages and postoperative complications. In this context, organoid technology in the field of regenerative medicine brings new hope for kidney disease treatment.
Recently, a study published in Biofabrication titled Microfluidic bioprinting as a tool to produce hiPSCs-derived renal organoids successfully constructed functional kidney organoids derived from human induced pluripotent stem cells (hiPSCs), laying an important foundation for kidney disease modeling, drug screening, and future regenerative therapies.
1. Core Technology: Directed Differentiation and Bioprinting from Stem Cells to Organoids
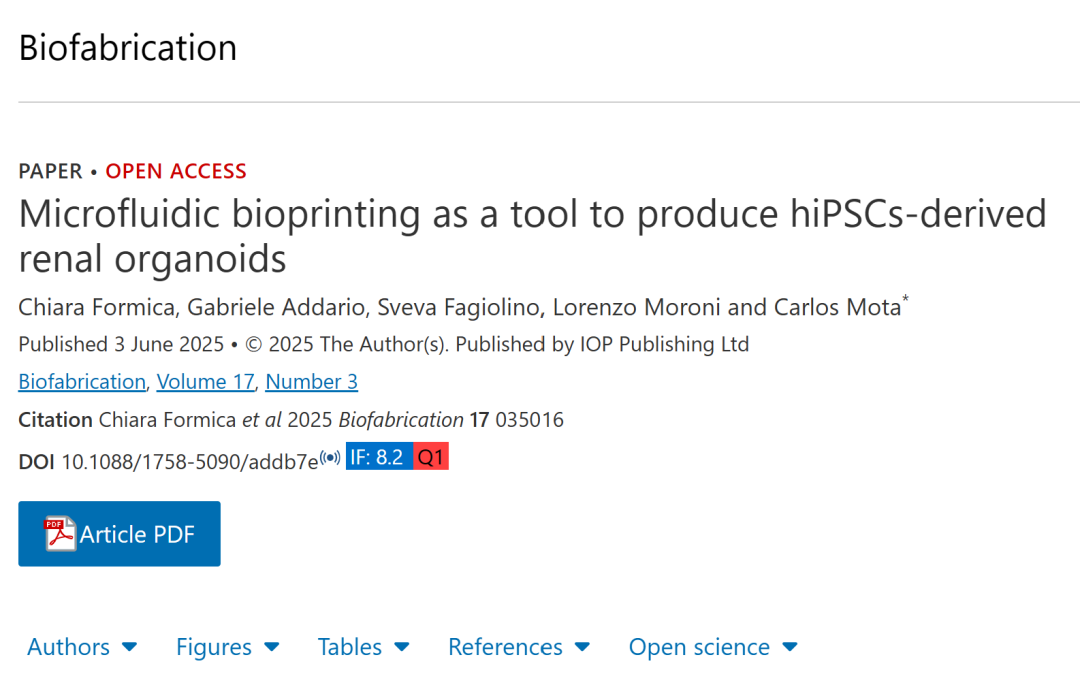
The research team first optimized the differentiation protocol of hiPSCs into renal progenitor cells. By sequentially adding specific growth factors (such as CHIR99021, noggin, FGF9, etc.), hiPSCs were induced to differentiate into metanephric mesenchyme (MM) and ureteric bud (UB) progenitor cells. Among them, MM progenitor cells expressed key markers such as SIX2 and PAX2, while UB progenitor cells exhibited characteristic expressions of GATA3 and CK8, confirming the effectiveness of the differentiation.
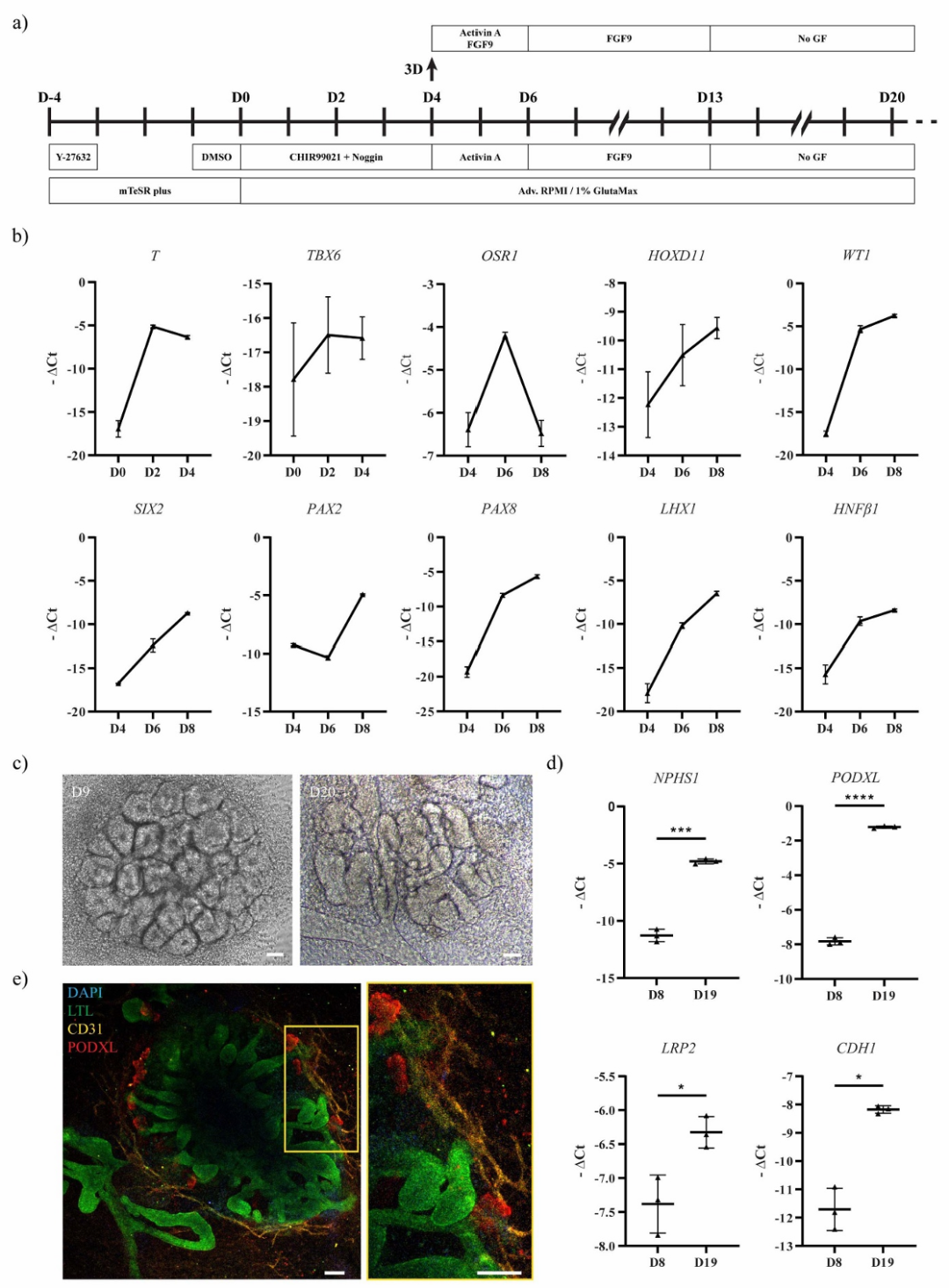 Figure 1. Generation and characterization of MM organoids
Figure 1. Generation and characterization of MM organoids
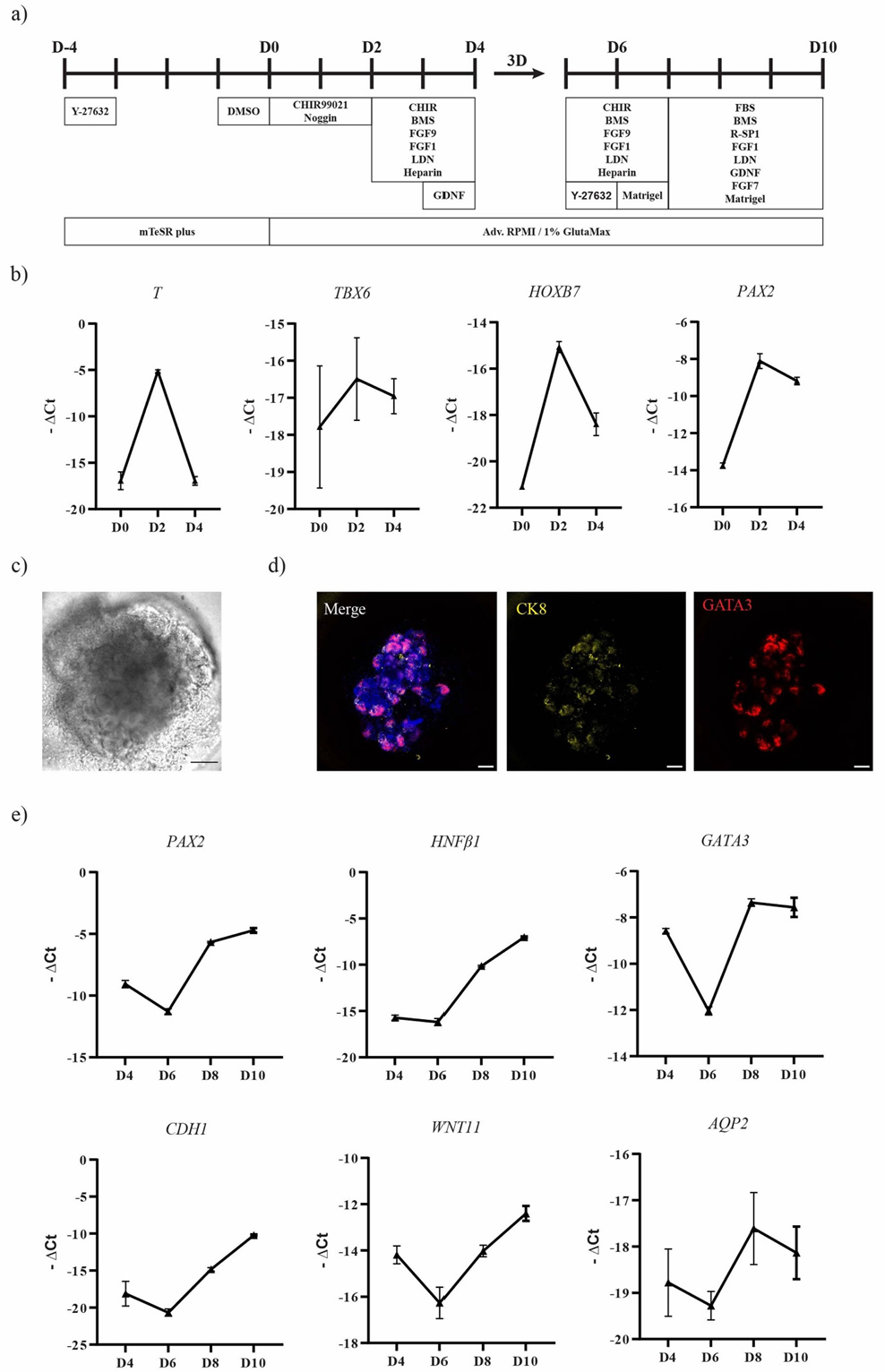 Figure 2. Generation and characterization of UB organoids
Figure 2. Generation and characterization of UB organoids
Subsequently, the researchers used microfluidic bioprinting technology to mix MM and UB progenitor cells in a 1:1 ratio, preparing a gelatin-containing core bioink, which was encapsulated in an alginate shell to form a core-shell structured printing filament. This printing method not only ensured cell viability in a low shear stress environment (live/dead staining showed a cell survival rate of over 90% after 24 hours) but also achieved precise control of filament diameter (average outer diameter 662.51±97.10 μm) through controllable pressure parameters (core/shell pressure set at 100 mbar).
2. Organoid Function Validation: Structural Maturity and Drug Response Capability
After being cultured in an optimized medium for two weeks, the printed filament exhibited significant kidney unit-like structure formation capability:
Morphological and Molecular Characteristics: On day 7, glomerular structures were visible, and on day 14, immunostaining showed typical kidney unit segmentation—PODXL-positive glomeruli, LTL-positive proximal tubules, and GATA3-positive collecting duct-like structures arranged in an orderly manner, resembling the spatial pattern of embryonic kidney development.
Functional Assessment: Through fluorescently labeled albumin uptake experiments, it was confirmed that the organoid proximal tubules possess filtration functions; further exposure to the nephrotoxic drug doxorubicin (2-10 μM) resulted in significant downregulation of glomerular marker PODXL, proximal tubule markers LRP2 and CDH1, while the kidney injury marker KIM1 was upregulated, indicating its ability to realistically simulate drug-induced kidney injury responses.
Automation and Standardization Advantages: Compared to traditionally manually prepared organoids, bioprinting technology significantly enhances the consistency between batches of organoids by precisely controlling cell spatial distribution and microenvironment, avoiding the variability of manual operations, and providing a reliable model for large-scale drug screening.
 Figure 3. Bioprinting MM+UB progenitor cells triggering organoid formation and functional studies
Figure 3. Bioprinting MM+UB progenitor cells triggering organoid formation and functional studies
3. Innovation and Application Prospects of Bioprinting Technology
The core advantage of microfluidic bioprinting lies in its ability for “multi-material co-assembly”: the gelatin in the core layer provides a low-viscosity, high biocompatible microenvironment for cells, supporting progenitor cell self-aggregation and differentiation; the alginate shell forms a physical barrier through calcium ion crosslinking, maintaining structural stability while allowing nutrient diffusion. This design not only replicates the interactions between cells and between cells and the matrix during kidney development but also reserves space for the subsequent introduction of endothelial cells, immune cells, and other components to construct more complex organoid systems.
Compared to traditional suspension culture, the automated characteristics of bioprinting technology make it more promising for large-scale production. Studies show that the printed progenitor cells can complete spatial rearrangement within 62 hours—MM cells gradually encapsulate UB cells, simulating the branching morphogenesis process of embryonic kidney, confirming the ability of this technology to reproduce organ development dynamics in vitro.
Conclusion
This study successfully constructed the most realistic kidney organoid model to date by integrating stem cell differentiation, biomaterials engineering, and precision printing technology. Its significance lies not only in providing a reproducible in vitro platform for kidney disease research but also in exploring an automated manufacturing pathway from “cell-material-structure” to functional organ substitutes. Although there is still a long translational period before clinical application, the potential of this technology in drug toxicity screening, personalized disease modeling, and tissue engineering is already evident. With the iteration of bioprinting technology and interdisciplinary integration, it may one day truly realize the medical vision of “printing kidneys to restart life,” bringing hope for cures to millions of kidney disease patients.
References:
Formica C, Addario G, Fagiolino S, Moroni L, Mota C. Microfluidic bioprinting as a tool to produce hiPSCs-derived renal organoids. Biofabrication. 2025;17(3):10.1088/1758-5090/addb7e. Published 2025 Jun 3. doi:10.1088/1758-5090/addb7e
This article is for academic sharing only. Please indicate the source when reprinting. If there is any infringement, please contact WeChat: bioonSir for deletion or modification!
Click below 「Read the original text」, visit the Bio Valley official website for more biological-related information~