
【Research Background】
Lithium metal batteries are one of the development directions for high specific energy batteries, attracting significant attention in both academia and industry. However, it is commonly believed that lithium metal electrolytes with excellent performance should use single-phase electrolytes, which often conflicts with the different properties of the anode and cathode. Therefore, the rational design of electrolytes based on the differences in properties of the anode and cathode is crucial.
Based on this, a research paper titled “Starving Free Solvents: Toward Immiscible Binary Liquid Electrolytes for Li-Metal Full Cells” was published in Adv. Funct. Mater. by Sang Kyu Kwak from Korea University and Sang-Young Lee from Yonsei University. The authors designed a type of immiscible binary liquid electrolyte (BLE) by “starving” the free solvent molecules in the electrolyte (i.e., enriching the coordinating solvent). This unique structure eliminates the need for solid electrolytes, thick separators, and gelation, which helps improve energy density. This work provides theoretical guidance and important references for the design of multifunctional electrolytes.
【Illustrated Guide】
To achieve the separation of two different liquid electrolytes, it is crucial to weaken the intermolecular interactions between free solvent molecules in the electrolyte. This indicates that promoting Li+ solvation (leading to a lack of free solvent molecules) will effectively facilitate the phase separation of the two electrolytes. In this study, the coordination number (CN) of the solvation structure is shown in Figure 1a. By integrating the radial distribution function (Figure 1b), the authors estimated the relative fractions of solvation species in the first solvation shell, assuming that the total average CN for Li+ (CN (Li+−solvent) + CN (Li+−anion)) is 5. Solvent-separated ion pairs (SSIP) (CN = 5) were observed only in electrolytes containing LiFSI [LiFSI/SN(1/5)(14.5%) and LiFSI/DME(1/5)(7.5%)]. By studying the solvation structures of SN- and DME-based electrolytes, their theoretical results were verified (Figure 1c). The Raman spectrum of SN-based electrolytes (Figure 1c, left) shows that, compared to lithium triflate (LiOTf) (or LiBF4)/dinitrile (SN) (1/5), LiFSI/SN(1/5) exhibits the highest proportion of the coordinating nitrile characteristic peak (≈2280 cm−1). Adding LiFSI to the mixed solvents of SN-DME resulted in the phase separation of SN- and DME-based electrolytes, while other salt-containing electrolytes remained miscible (Figure 1d). Meanwhile, the SN-based electrolyte shifts more negatively than the DME-based electrolyte due to the higher solvation ability of SN (ε = 54) compared to DME (ε = 7.2). This result was confirmed by using MD simulation to calculate the solvation free energy of the electrolyte (Figure 1f). With the enhancement of salt dissociation, the absolute value of the solvation energy (ΔGsolv) of the electrolyte gradually increases, proving that dissociated ions tend to exhibit stronger Li+−solvent interactions. Compared to adding other salts (LiOTf and LiBF4), adding LiFSI leads to a more negative ΔGsolv, consistent with the Raman (Figure 1c) and 7Li NMR results (Figure 1e).
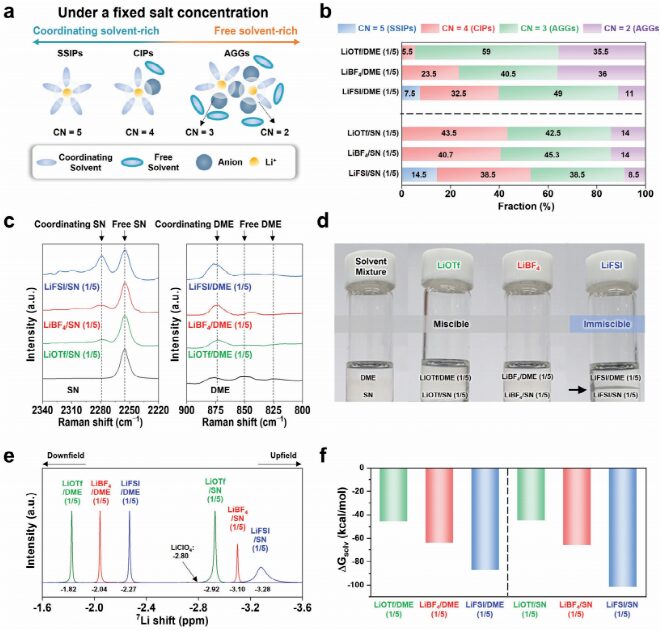
Figure 1 Analysis of the solvation structure of the electrolyte.
The results of the Raman spectrum (Figure 2a) indicate that, with the participation of FSI− in the solvation sheath, most solvent molecules exist in a highly coordinated state. The solvation structure was further studied by calculating the structure factor [S(k)] of the electrolyte (Figure 2b). After mixing for one minute, both the dilute electrolyte (LiFSI/SN(1/5)−LiFSI/DME(1/5)) and concentrated electrolyte (LiFSI/SN(1/0.8)−LiFSI/DME(1/1.5)) exhibited phase separation, and the distinct liquid-liquid interface between SN- and DME-electrolytes confirmed this (Figure 2c). Additionally, it was difficult to detect SN molecules in the upper phase Raman spectrum of the electrolyte pair (Figure 2d), where the upper phase electrolyte is LiFSI/DME(1/5) (dilute) and LiFSI/DME(1/1.5) (concentrated). By analyzing the intermolecular energy (ΔEint) of its components, the persistent phase separation behavior in concentrated electrolyte was further elucidated (Figure 2e). The favorable effect of high salt concentration on the phase separation of the electrolyte pair is shown in Figure 2f. Notably, the increase in intermolecular binding energy (ΔEint) indicates that the coordination of Li+-FSI– in the solvation sheath increases, contributing to further strengthening the phase separation of the concentrated electrolyte.
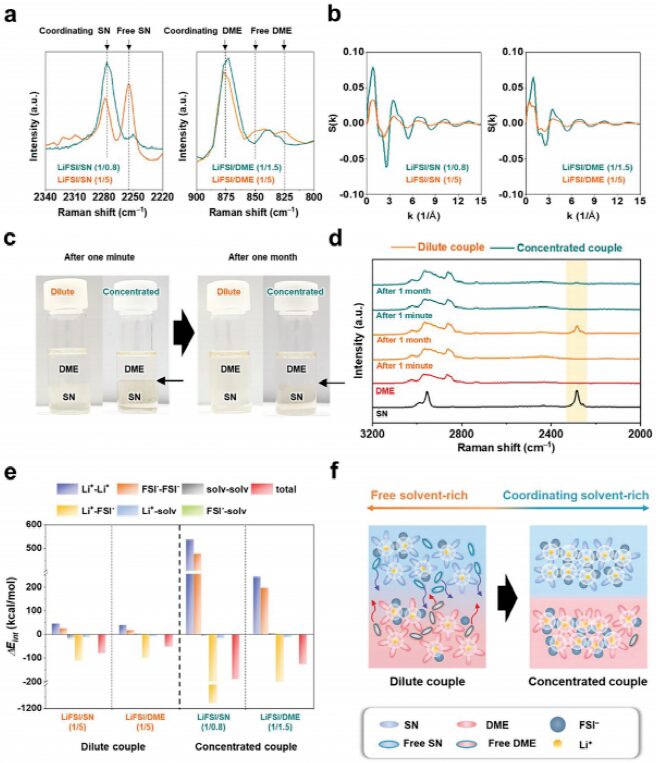
Figure 2 Regulation of electrolyte phase separation by LiFSI.
In Figure 3, the ionic conductivity of the binary liquid electrolyte (BLE) and its components (LiFSI/SN (1/0.8; referred to as SN-EL) and LiFSI/DME (1/1.5; referred to as DME-EL)) is detected as a function of temperature (Figure 3a). The electrochemical stability of BLE was studied using linear sweep voltammetry (LSV) on stainless steel electrodes (Figure 3b). SN-EL exhibits high oxidative stability above 5 V but insufficient reductive stability around ≈1 V. DME-EL shows the opposite results (good reductive stability around 0 V and poor oxidative stability around 4 V). In addition to the LSV results, the authors also studied the lithium plating/stripping behavior of BLE components (SN-EL and DME-EL) using Li||Cu asymmetric batteries at a current density of 1.0 mA cm -2 and areal capacity of 1.0 mAh cm -2 (Figure 3c). DME-EL shows high Coulombic efficiency (≈99%) over 100 cycles, demonstrating stable plating/stripping cycling performance, while SN-EL shows low Coulombic efficiency (≈20%), indicating poor performance, which does not show significant differences with the battery resistance between BLE and DME-EL (Figure 3d).
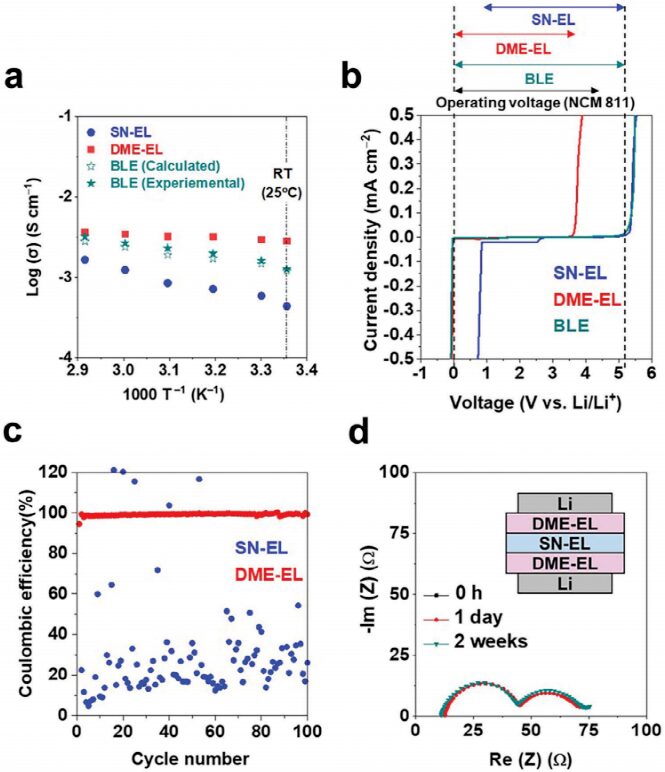
Figure 3 Electrochemical characterization of binary electrolytes.
The authors assembled LiFSI/SN (1/0.8; SN-EL) and LiFSI/DME (1/1.5; DME-EL) with a lithium metal anode (thickness = 50µm) and an NCM811 cathode (areal capacity = 2.5 mAh cm-2) to explore their suitability as electrolytes for lithium metal full cells (Figure 4a). As previously mentioned, the poor reductive stability of SN-EL continuously leads to the decomposition of the lithium metal anode electrolyte, resulting in irreversible stripping/plating of lithium, ultimately leading to battery failure, as shown in Figure 4b (left). In contrast, the battery using BLE shows the highest cycling stability (capacity retention of 88% after 200 cycles) due to the customized compatibility of the electrolyte-electrode (both anode and cathode) achieved by the introduction of SN-EL and DME-EL (Figure 4b, right). To further elucidate the impact of BLE, the authors prepared a high-concentration electrolyte (LiFSI/DME(1/1.15)), with the LiFSI salt concentration comparable to that of BLE, and studied its battery performance (Figure 4c). Compared to DME-EL and LiFSI/DME(1/1.15), the organic and inorganic CEI components in NCM 811 cycled with BLE are significantly less, indicating that the oxidative decomposition of the solvent and the dissolution of transition metals in BLE are highly suppressed. These results suggest that simply increasing salt concentration is insufficient to achieve good cycling performance, while also demonstrating the feasible role of immiscible BLE as customized electrolytes for electrodes.
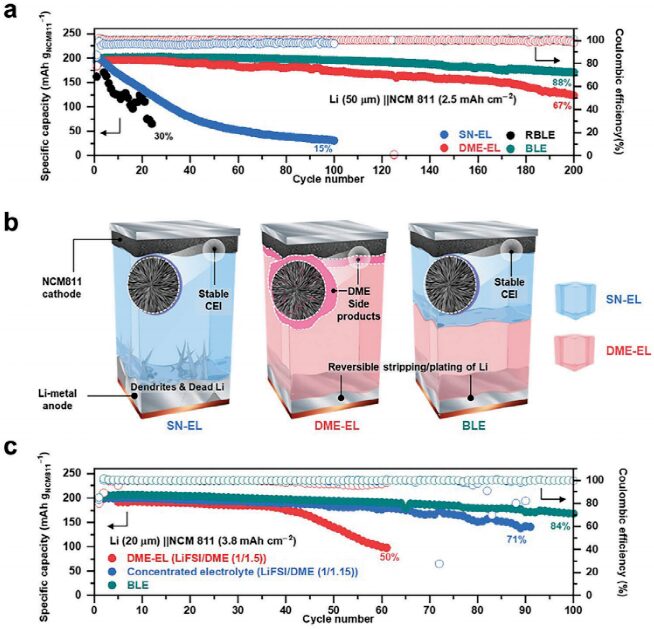
Figure 4 Electrochemical performance testing of Li||NCM811 batteries.
To better understand the role of BLE in cycling stability, the authors performed XPS characterization of the CEI of the Li(50µm)||NCM811 (2.5 mAh cm−2) battery after 50 cycles (Figure 5). These results indicate that SN-EL in BLE (facing the NCM811 cathode) plays a feasible role in forming a stable CEI. Subsequently, the cycled lithium metal anode was studied using scanning electron microscopy and XPS analysis. Dense and uniform lithium layers were observed in the lithium metal cycled with dioxane-EL and BLE, while irregular and porous morphologies were observed in the lithium metal cycled with SN-EL. Structural characterization of the cycled lithium metal anode indicates that the DME-EL in BLE (adjacent to the lithium metal anode) is beneficial for stabilizing SEI formation. This suggests the electrochemical feasibility of BLE in simultaneously stabilizing the lithium metal anode and the high-voltage NCM811 cathode, thereby contributing to superior full battery performance, which is not easily achievable with single-phase liquid electrolytes.
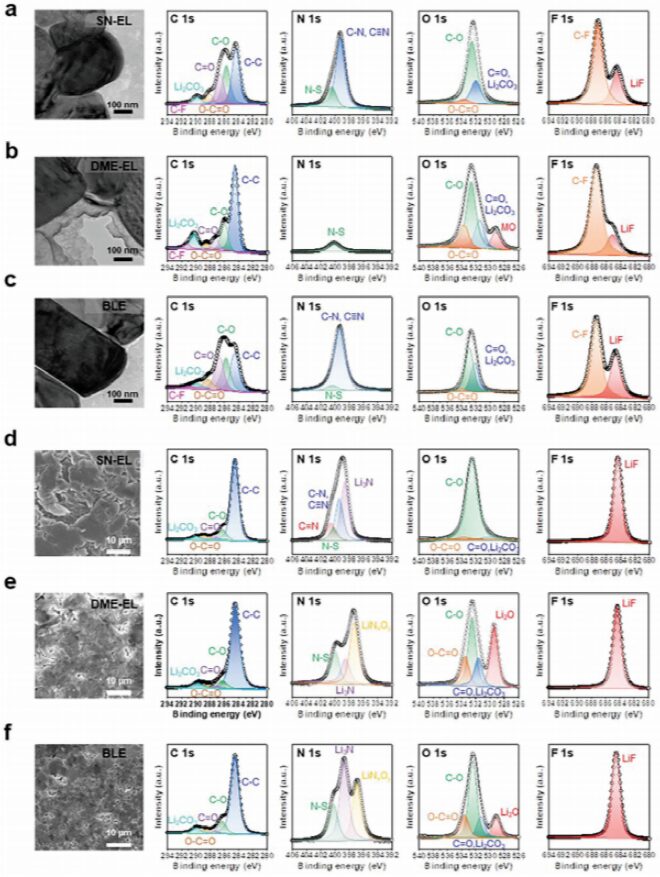
Figure 5 XPS analysis of the battery anode and cathode after cycling.
【Summary and Outlook】
In summary, the authors designed BLE by lacking free solvent molecules (i.e., rich coordinating solvent) in the electrolyte. BLE is used to simultaneously stabilize the lithium metal anode and NCM811 cathode for practical high-voltage lithium metal full batteries. Among the lithium salts studied in this paper (LiOTf, LiBF4, and LiFSI), LiFSI promotes the solvation of Li+ due to its high dissociation ability, leading to phase separation of the initially miscible solvent mixture (SN-DME). At higher concentrations of LiFSI, this phase separation behavior becomes more pronounced, further reducing free solvent molecules. Additionally, increasing the concentration of LiFSI enhances the coordination of Li+−FSI− in the solvation sheath, thereby facilitating the formation of a fluorinated electrode-electrolyte interface. The BLE concept proposed in this study is a promising electrolyte strategy. Furthermore, BLE can be easily designed without the need for serious consideration of new electrolyte chemistry, serving as a universal and scalable platform technology to promote its application in various emerging batteries.
Hyunseok Moon, Gwan Yeong Jung, Ji Eun Lee, Imanuel Kristanto, Sang Kyu Kwak*, Sang-Young Lee*. Starving Free Solvents: Toward Immiscible Binary Liquid Electrolytes for Li-Metal Full Cells. Adv. Funct. Mater. 2023, 2302543.
https://onlinelibrary.wiley.com/doi/full/10.1002/adfm.202302543