Summary
This review systematically examines the structural classification, defect construction methods, and functional applications of titanium-based metal-organic frameworks (Ti-MOFs). First, the authors elaborate on the importance of defect engineering in regulating the properties of solid materials and point out that Ti-MOFs are difficult to synthesize and introduce defects due to the strong oxygen affinity of Ti⁴⁺ and its tendency to hydrolyze into TiO₂, but these characteristics endow Ti-MOFs with excellent photocatalytic and redox activity. Subsequently, based on the dimensions of Ti-oxo clusters (SBUs) (0D, 1D, 2D), a systematic classification of existing Ti-MOFs is conducted, revealing the structural diversity of various TiO₆ units, TiₓOᵧ multinuclear clusters, and mixed metal clusters. Next, eight major defect construction strategies are outlined, including modulator-assisted synthesis, post-synthetic treatment, light/thermal activation, soft ligands, solvent/precursor regulation, templating methods, alloying, and redox control, providing a roadmap for the precise introduction of coordination unsaturated sites. The article further summarizes the significant performance enhancements of defect Ti-MOFs in photocatalysis, gas adsorption and separation, electrocatalysis, and heterogeneous catalysis. Finally, the authors look forward to the future: the need to develop controllable Ti precursors and new cluster structures, combined with in situ characterization and computational simulations, to achieve an overall transition from “revelation” to “precise construction,” laying the foundation for the industrial application of high-performance Ti-MOFs.
Author Team

Research Background
The Importance of Defects in Materials: Defects are widely present in solid materials and can significantly affect their optical, electrical, magnetic, acoustic, mechanical, and thermal properties. By purposefully designing and regulating defects (defect engineering), the reactivity and functionality of materials can be altered, leading to breakthroughs in catalysis, electronic devices, photonics, and energy storage.
Defects in MOFs: Metal-organic frameworks (MOFs) are a class of porous crystalline materials formed by the ordered coordination of metal clusters (or ions) with organic linkers, characterized by high specific surface area and tunable pore size. Naturally occurring defects (missing linkers, missing metal clusters, etc.) can significantly affect the stability and performance of MOFs. Extensive research on Zr-MOFs (such as UiO-66) has revealed how to enhance the number of open metal sites through defects, optimizing catalytic and separation performance.
Challenges and Opportunities of Ti-MOFs: Compared to Zr⁴⁺, Ti⁴⁺ has a stronger oxygen affinity and easily hydrolyzes to form TiO₂, leading to complex synthesis of Ti-MOFs. Ti-oxo clusters lack stable precursors, and the sensitivity of hydrolysis-condensation reactions often results in amorphous or mixed-phase products. Nevertheless, Ti-MOFs show potential in environmental remediation, CO₂ reduction, and water splitting due to their high photocatalytic and reversible redox activity. Systematic exploration of the structural classification and defect introduction strategies of Ti-MOFs will help overcome synthesis obstacles and achieve performance leaps.
Classification of Ti-MOFs by SBUs
Understanding the structural diversity of Ti-MOFs is fundamental for precise design and defect introduction. This section classifies existing Ti-MOFs based on the dimensions of secondary building units (SBUs) into five categories: 0D single Ti ion clusters, 0D multinuclear Ti-oxo clusters, mixed metal clusters, 1D Ti-oxo chains, and 2D Ti-oxo layers. By systematically sorting the structural units, ligand types, and topological features of representative materials, this aims to reveal how the diversity of Ti clusters provides rich possibilities for framework functionalization and defect engineering.
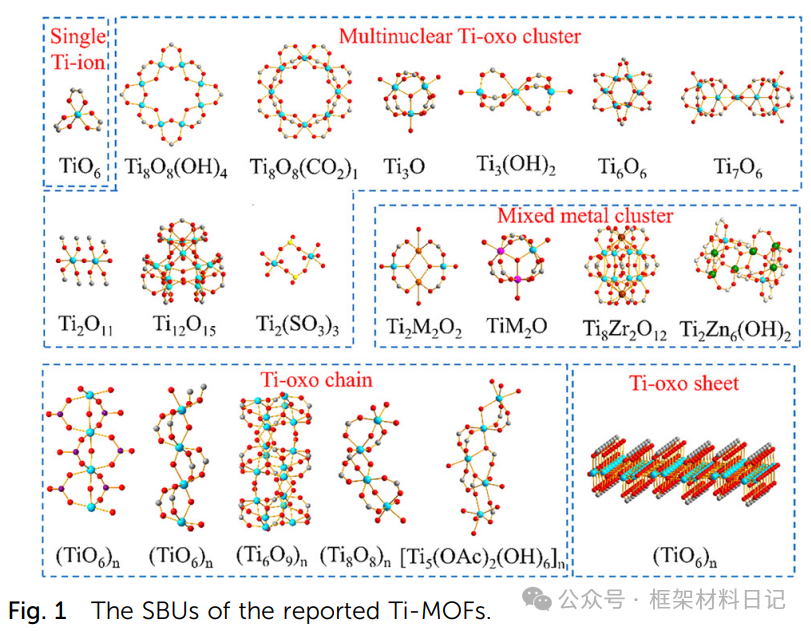
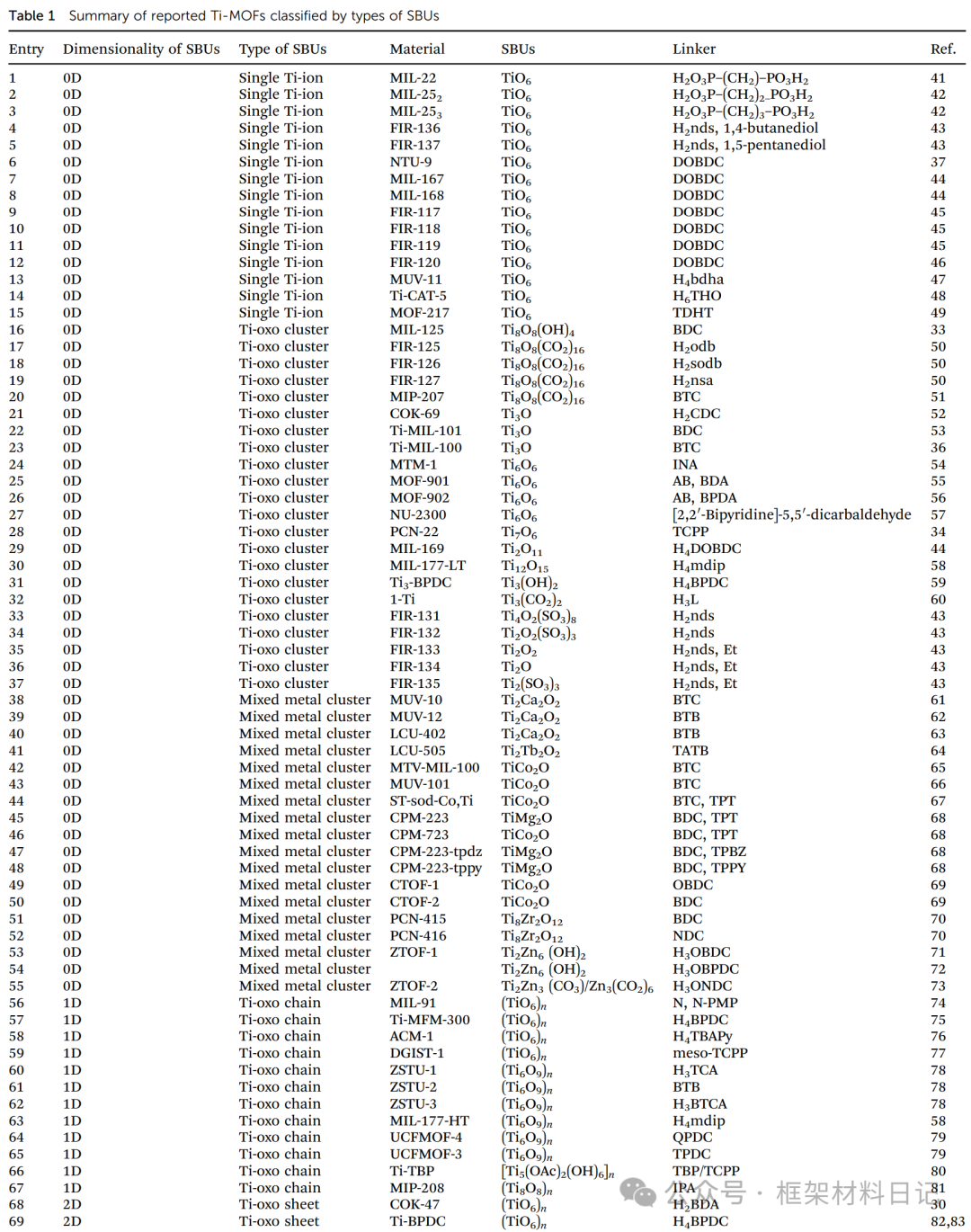
1. Single Ti Ion Constructed MOFs
Single Ti ion MOFs are constructed using TiO₆ octahedra and various organic ligands, showcasing diverse topologies of 0D clusters, 1D pillars, 2D layers, and 3D networks, providing refined design space for subsequent defect site insertion.
MIL-251/22/23 Series: Synthesized using di(alkyl) phosphate ligands and anhydrous TiO₂, forming TiO₆ units arranged in a pillar-like manner.
FIR-136/137: Using 1,5-naphthalenedisulfonic acid (nds) and diols to regulate coordination rates, resulting in 2D/sql and 3D/diamond topologies.
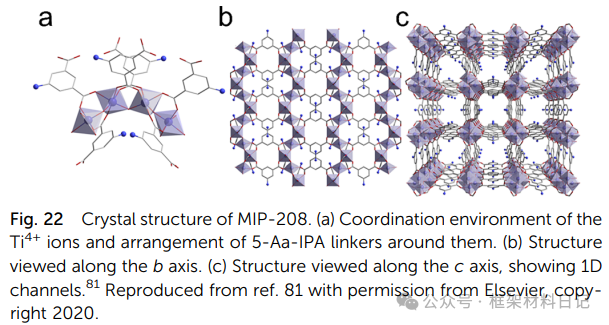
NTU-9 and MIL-167/168: Under H₂DOBDC ligand, TiO₆ clusters are assembled into 2D layered and 3D, 1D chain structures.
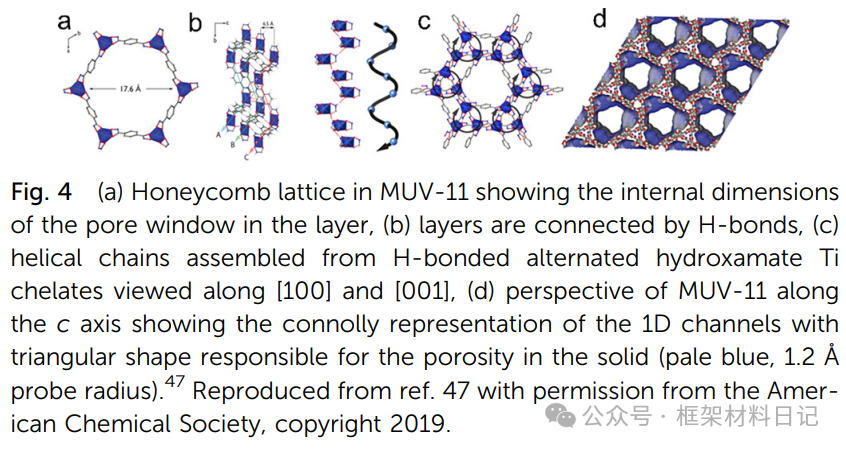
MUV-11: Hydroxamic acid ligands with Ti(IV) construct highly twisted honeycomb layers, pore size ~1.5×NTU-9.
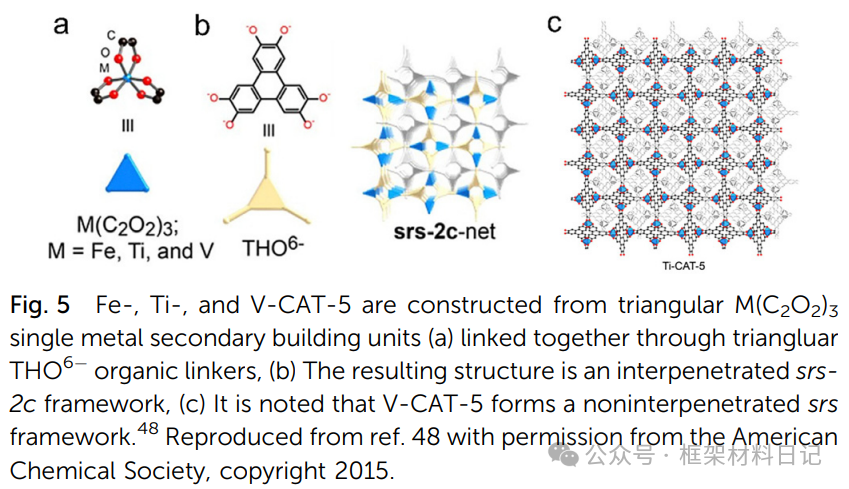
Ti-CAT-5 and MOF-217: Aromatic diols with Ti⁴⁺ form octa-TiO₆ clusters, resulting in interpenetrating srs topology.
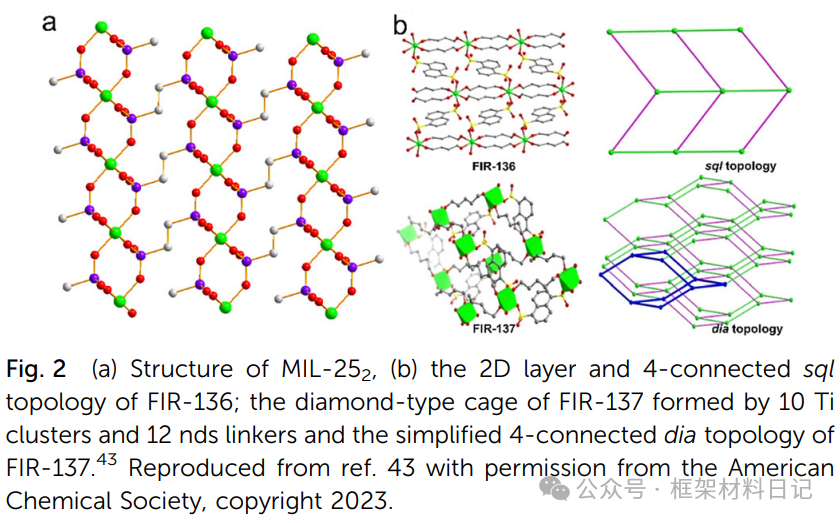
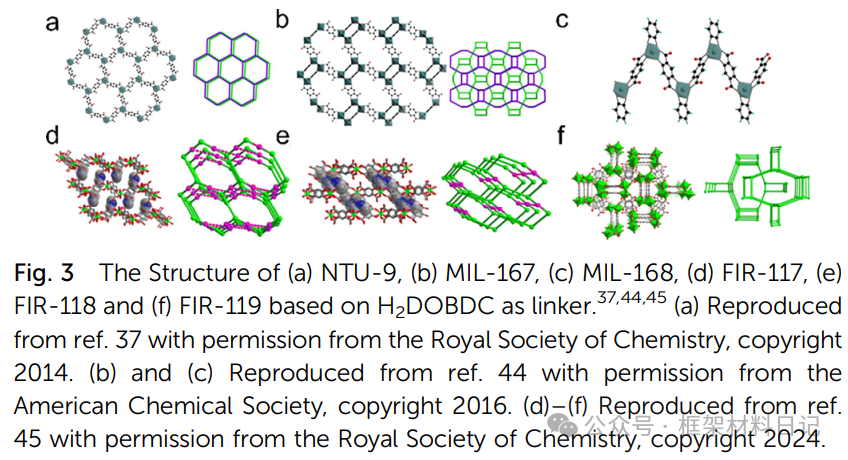
2. Multinuclear Ti-oxo Cluster Constructed MOFs
Multinuclear Ti-oxo cluster MOFs are constructed using TiₓOᵧ clusters as nodes, forming various topologies such as fcu, btc, and srs, showcasing adjustable Ti(III/IV) and large pore advantages, demonstrating excellent performance in photocatalysis and gas adsorption.
MIL-125 and FIR-125–127: Ti₈O₈(OH)₄ clusters stack in fcu arrangements, with diverse cavity structures; under light, they can generate Ti(III)–Ti(IV) mixed valence states, exhibiting color and photocatalytic capabilities.
COK-69(Ti₃O): Flexible M₃O clusters with carboxylic acids exhibit a “breathing” effect, capturing Ti(III) under UV light.
Ti-MIL-100/101: Ti₃O clusters with tricarboxylic acid ligands create large pore structures, where Ti(III) can generate superoxide/peroxide species with O₂, achieving high H₂ production efficiency.
MOF-901/902 and NU-2300: In situ Ti₆O₆ clusters with aldehyde groups are obtained through imine condensation, resulting in layered and topologically isomorphic MOFs with different pore sizes.
PCN-22h and MIL-169/177: Higher nuclearity Ti₇O₆, Ti₁₂O₁₅ clusters construct porous structures, with Ti–O condensation degrees reaching 1.25–1.5, approaching TiO₂ characteristics.
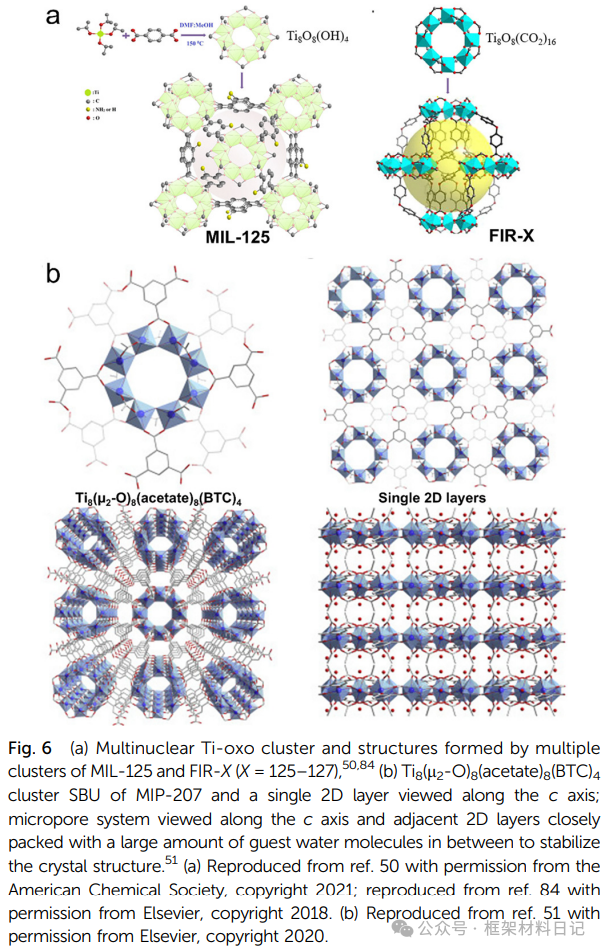
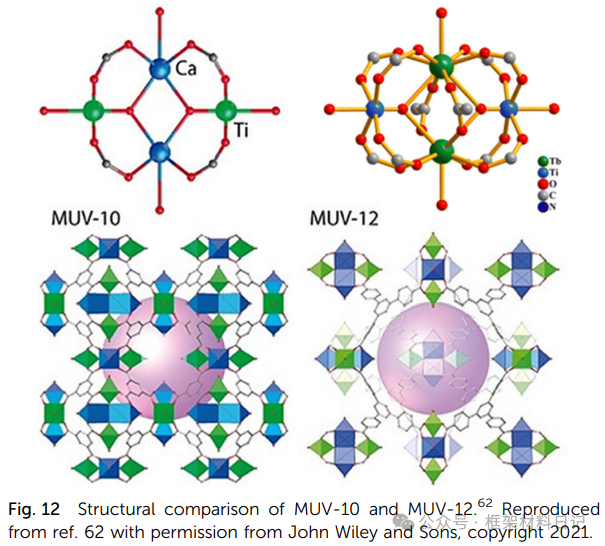
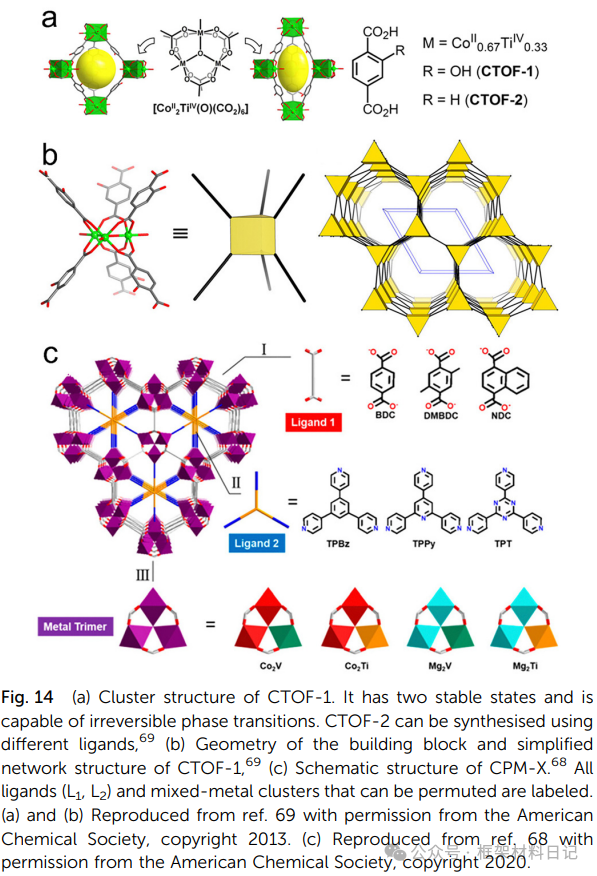
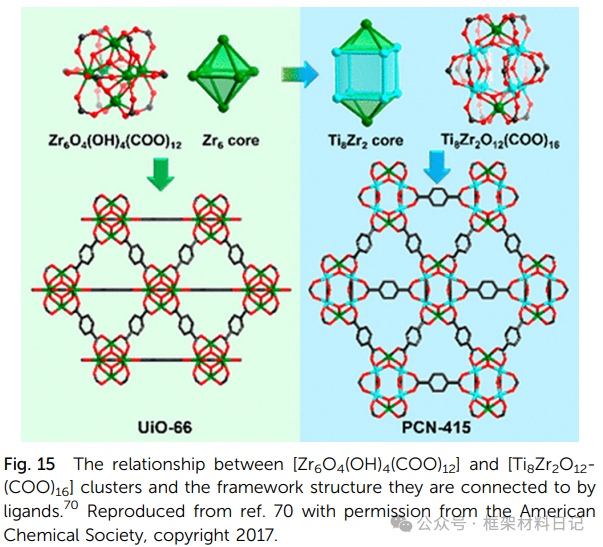
3. Mixed Metal Ti-MOFs
By introducing a second metal, mixed metal clusters in MOFs have unique advantages in stability, charge transfer, and light response regulation, providing new ideas for precise defect construction.
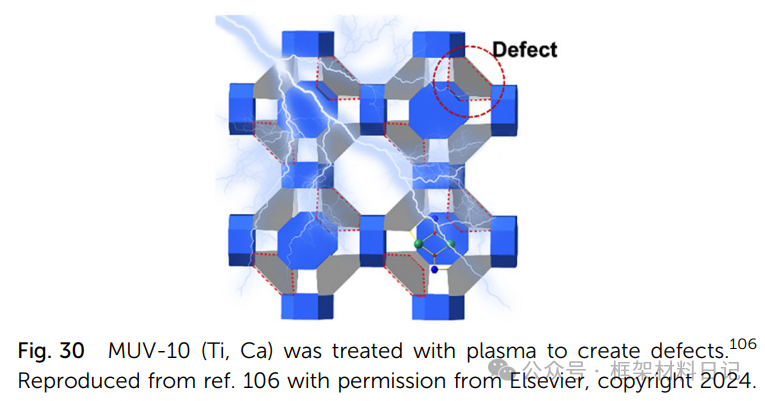
MUV-10/MUV-12 (Ti–Ca): Ti₂Ca₂ clusters with tricarboxylic acid or tricarboxylic phenyl ligands form a 3D neutral porous framework.
PCN-415/416 (Ti–Zr): Ti₈Zr₂ clusters with terephthalic acid/naphthalene dicarboxylic acid exhibit tunable photoluminescence and water-splitting activity.
MTV-MIL-100(Ti,M): TiM₂O clusters (M=Co, Ni, Mn, Fe) fine-tune the energy band, extending visible light response.
ST-sod-Co,Ti and CPM-223 series (Ti–Co/Mg): TiCo₂ forms sod or mpa topologies with various intercalated ligands, exhibiting thermal/chemical stability.
ZTOF-1/2 (Ti–Zn): Ti₂Zn₆ clusters or alternating Zn₃(CO₃)/Zn₃(CO₂) with phthalic/naphthalene dicarboxylic acids create innovative mixed metal topologies.




4. Ti-oxo Chain Structure MOFs
1D Ti–O chain MOFs possess continuous conductive/mass transfer channels, with structures flexibly regulated through clusters and -OH bridging, suitable for applications in electronics and selective catalysis.
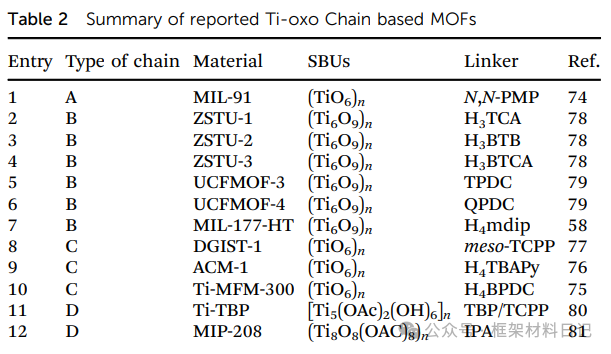
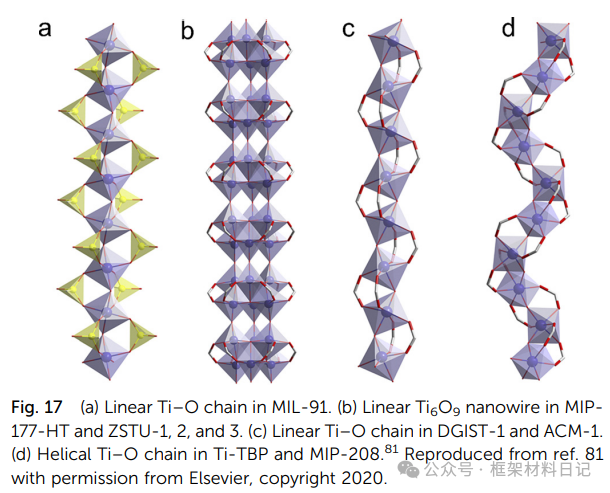
MIL-91(Ti): TiO₆ octahedra linked by phosphodiester form bending chains, creating a 3D hybrid network.
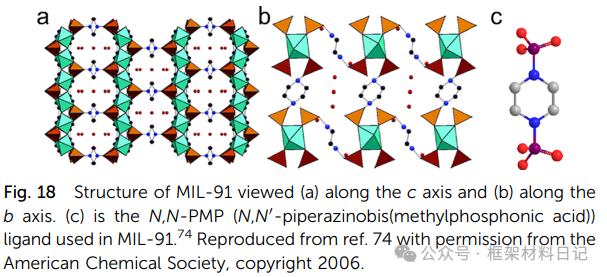
ZSTU-1/2/3 (Ti₆O₉)n: Ti₆O₆ clusters + m²-OH extend into nanowires, cross-linking with (3,6)-kdg layers, with pore sizes adjustable by ligand length.
UCFMOF-3/4: New TiO₂ chains stitched with phthalic acid derivatives exhibit conductivity and photocatalytic potential.
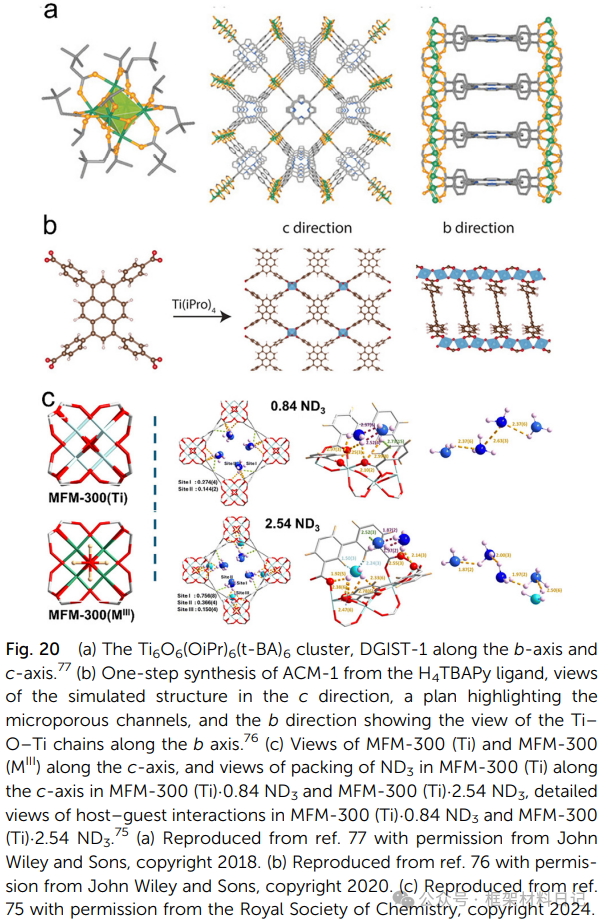
 5. Ti-oxo Layered Structure MOFs:2D Ti-O sheets combine controllable interlayer “spacing,” allowing for precise exposure of active sites after defect introduction, suitable for surface catalysis and molecular sieving.
5. Ti-oxo Layered Structure MOFs:2D Ti-O sheets combine controllable interlayer “spacing,” allowing for precise exposure of active sites after defect introduction, suitable for surface catalysis and molecular sieving.
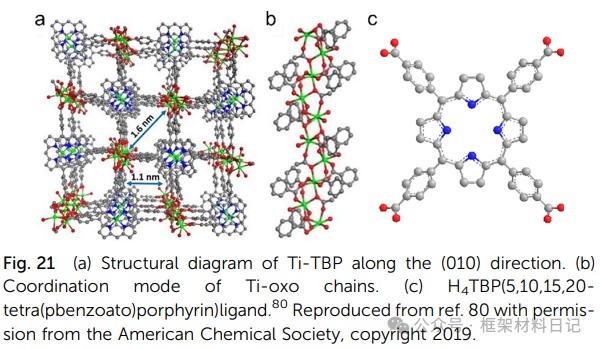
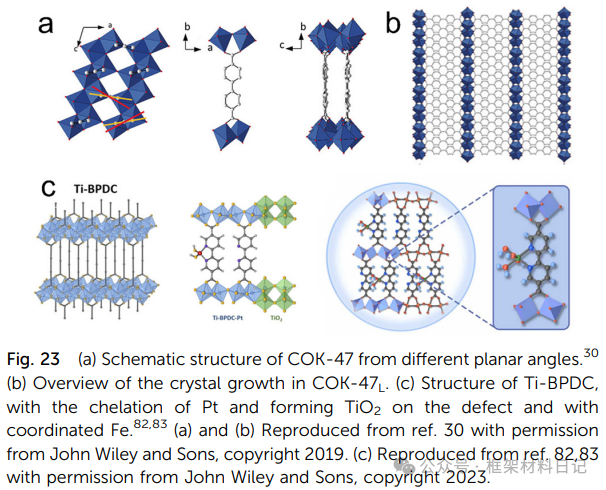
COK-47 and Ti-BPDC: TiO₆ units spread in a plane forming layers, with interlayer π–π or H-bond interactions, exhibiting high rigidity and allowing for the insertion of various molecules.
Defect Introduction Strategies
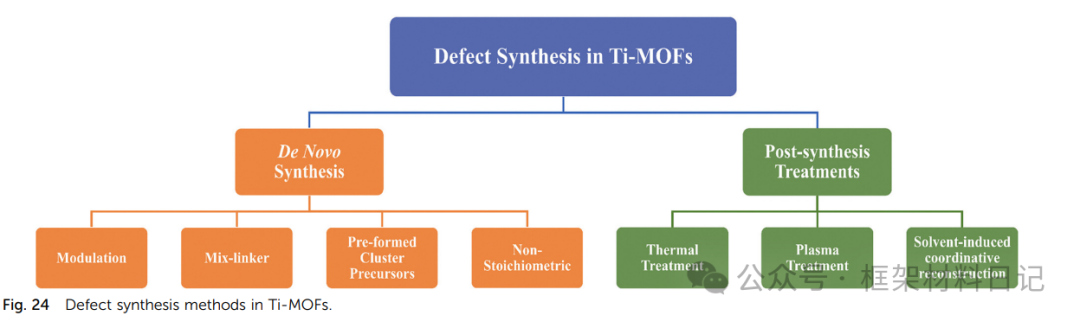
Ti-MOFs are inherently coordination-saturated, making it difficult to directly expose active sites; however, purposefully constructing coordination vacancies can significantly enhance performance. This section reviews eight mainstream defect construction methods—from modulators and pre-synthesized cluster strategies during the synthesis phase to post-treatment methods such as thermal, plasma, and solvent reconstruction, as well as ligand mixing and non-stoichiometric methods—and discusses their mechanisms, controllability, and applicability, providing a roadmap for the directional design of multi-scale and multi-type defects.
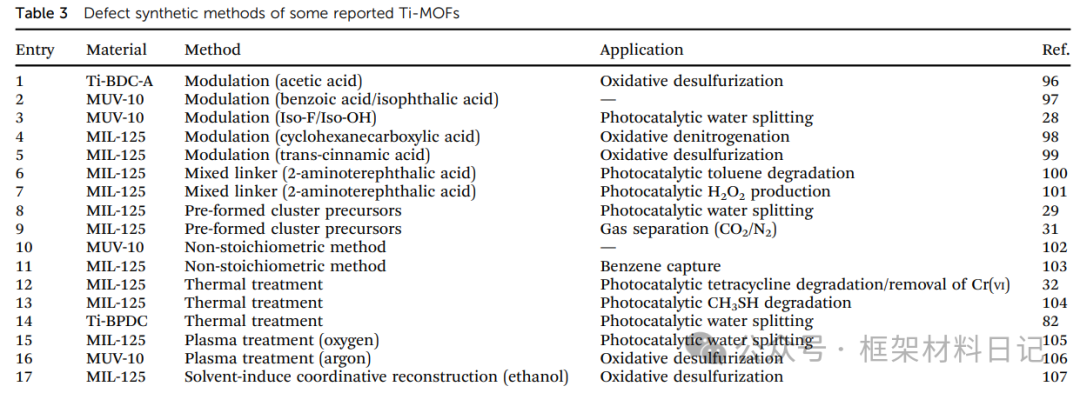
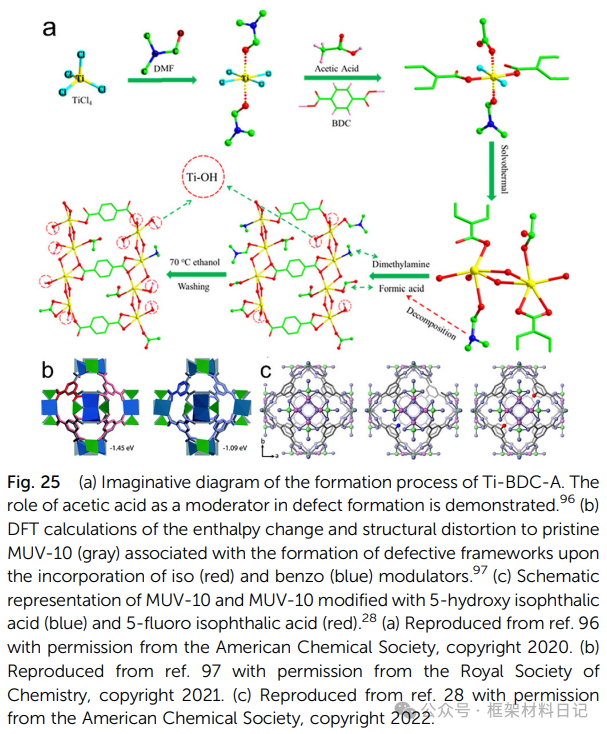
1. Modulator-Assisted Synthesis: Adding competitive ligands such as pyridine and acetic acid to achieve the “ligand competition” that results in the absence of organic ligands or metal clusters.
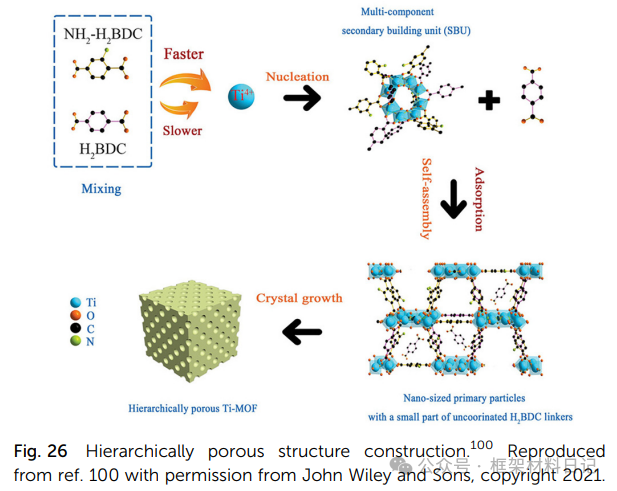
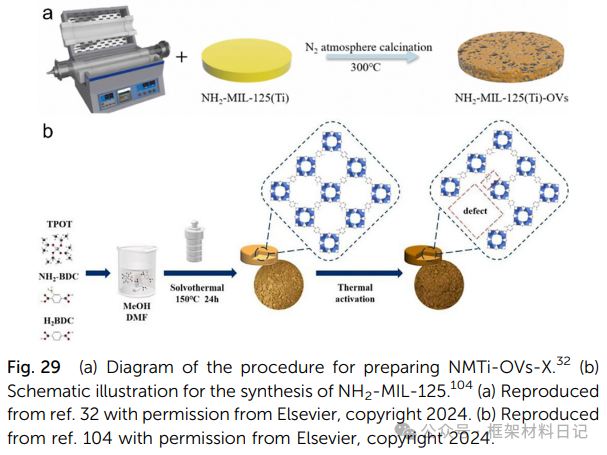
2. Mixed Ligand Method: For example, incorporating 2-amino-terephthalic acid into NH₂-MIL-125 generates ligand vacancies and small pores.
3. Pre-synthesized Cluster Precursors: Using precursors like Ti₈Ph to lower crystallization temperatures and introduce metal site vacancies.
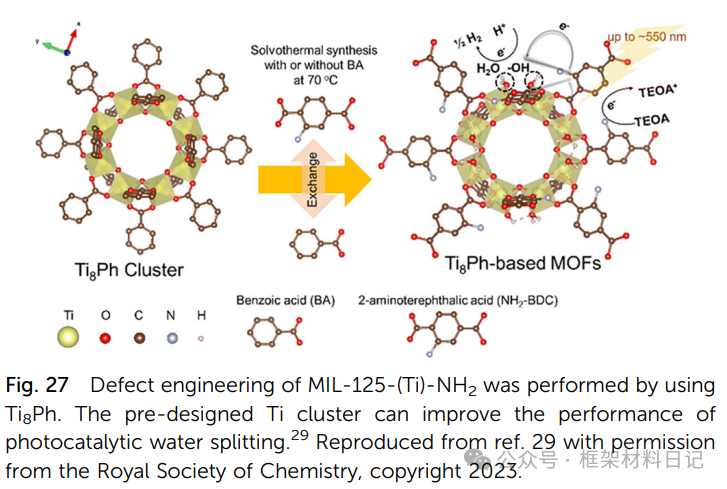
4. Non-stoichiometric Synthesis: Adjusting the metal/ligand ratio generates a disordered distribution of cluster defects and ligand defects.
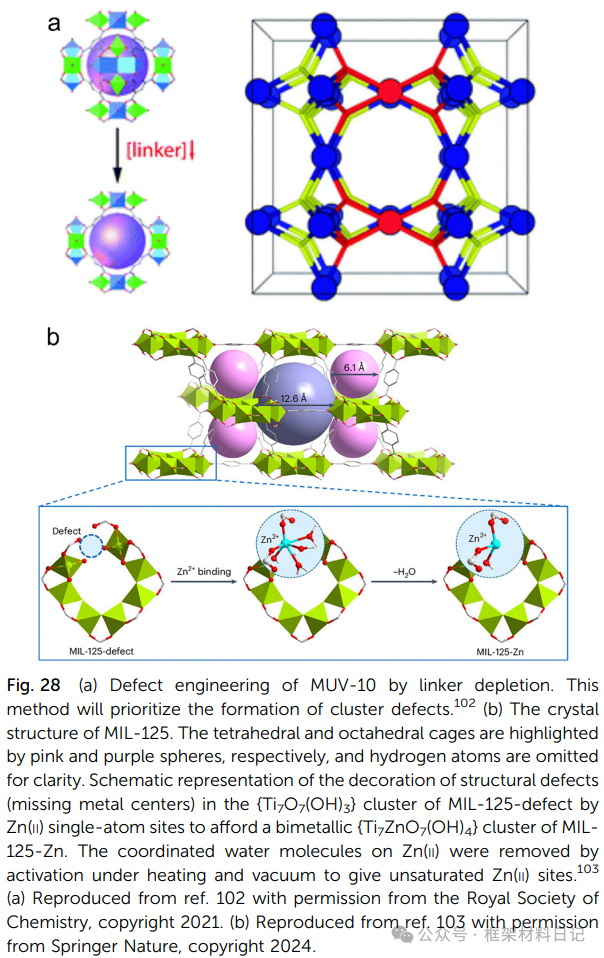
5. Thermal Treatment: Controlled pyrolysis forms vacancies from ligand degradation and oxygen vacancies.
6. Plasma Treatment:: O₂/Ar plasma bombardment removes ligands, rapidly generating high concentrations of defects.
7. Solvent-Induced Reconstruction:: For example, treating MIL-125-Ti-ATA with ethanol forms hollow nanosheets through esterification and ligand removal.
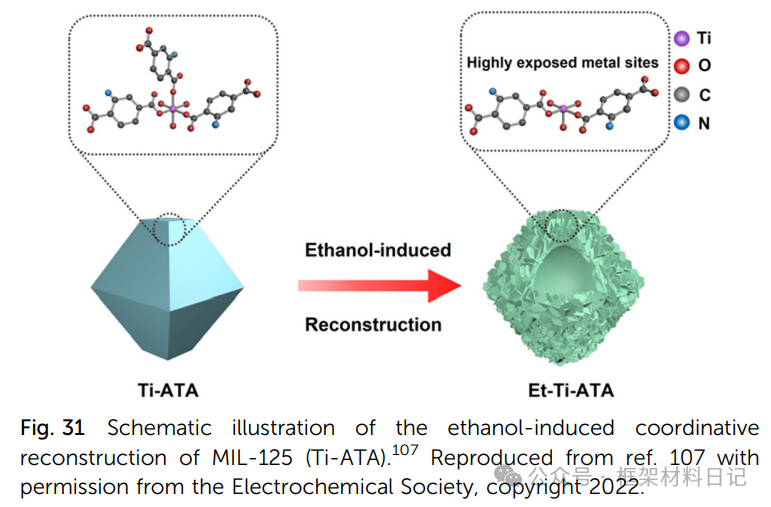
8. Redox Regulation:: Photochemical or chemical reduction generates Ti(III) centers, accompanied by the introduction of coordination unsaturation defects.
Applications of Defect Ti-MOFs
Defects not only alter the pore structure and electronic states of Ti-MOFs but also play a key role in various catalytic and separation processes. This section focuses on the latest advancements of defect Ti-MOFs in photocatalysis, water splitting and organic pollutant degradation, gas adsorption and separation, electrocatalysis, and heterogeneous catalysis, explaining how vacancy sites synergize with microporous networks to optimize photogenerated carrier separation, molecular diffusion, and active center exposure, thus driving the development of high-performance materials.
1. Photocatalysis
Defects enhance light absorption and carrier separation: Defect sites can form localized energy levels, narrowing the bandgap and enhancing visible light response. Ti(III) centers and open metal sites accelerate electron-hole separation and migration, such as the defect MIL-125 significantly improving the degradation rates of methylene blue and rhodamine B.
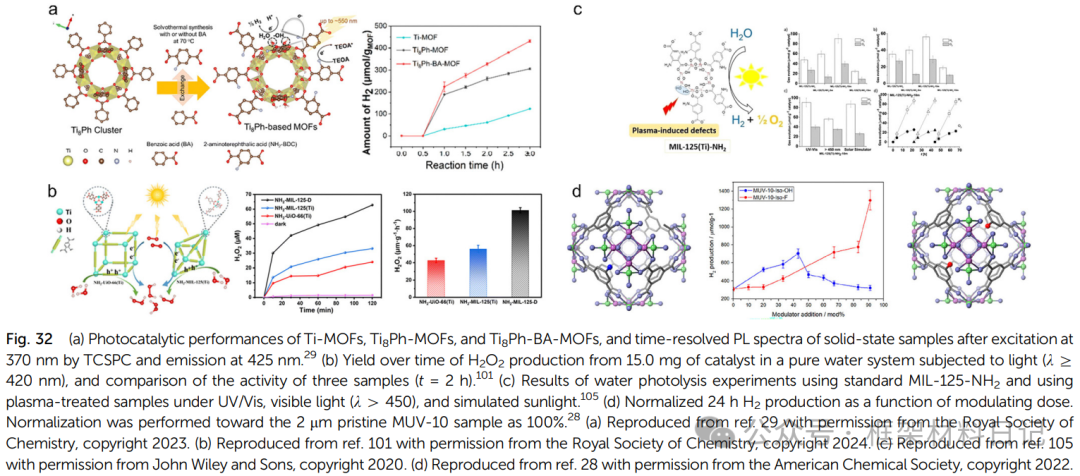
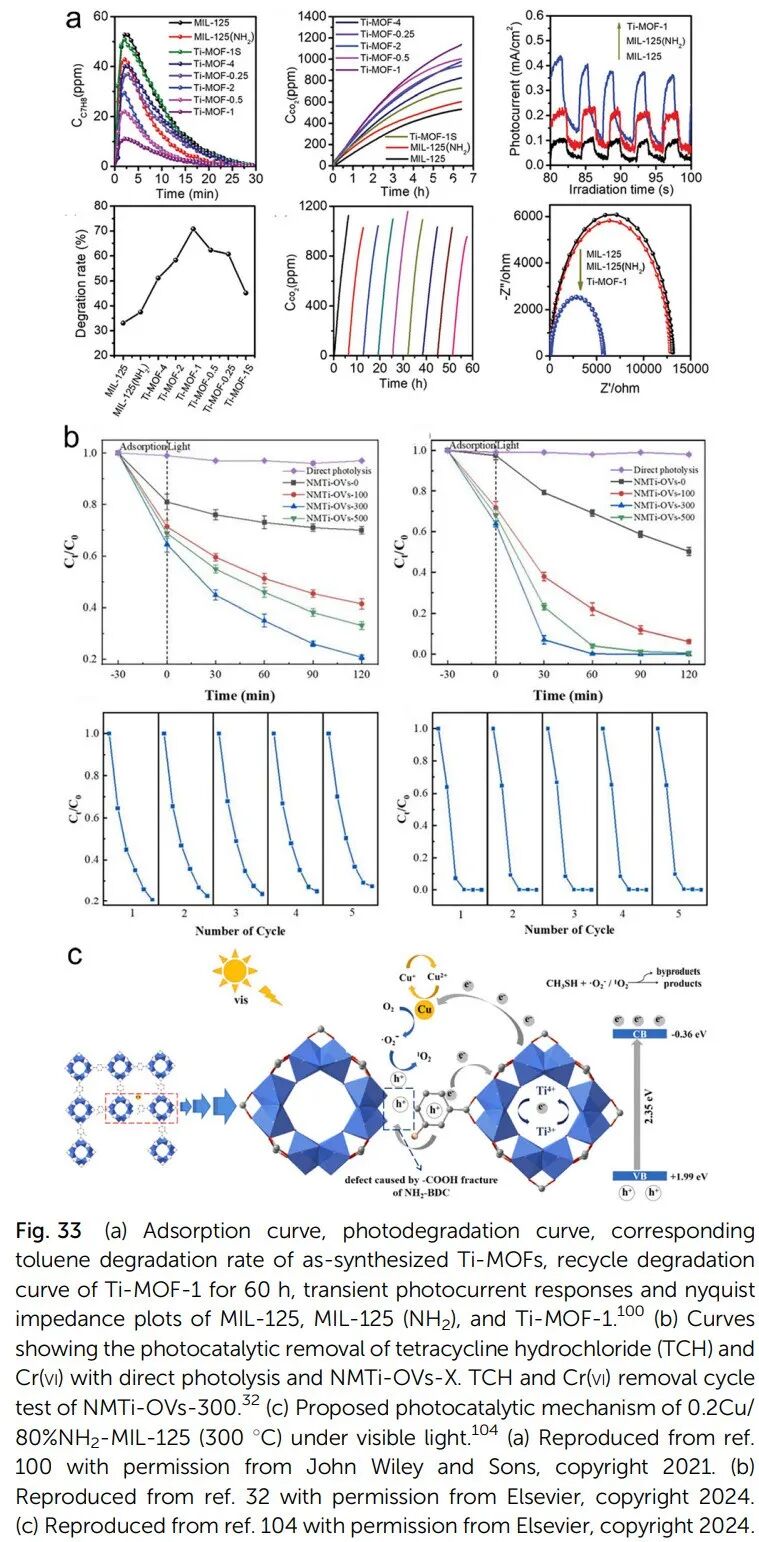
2. Organic Catalysis
Ti serves as a catalytic active center in organic catalytic reactions, with current research focusing on desulfurization, oxidizing large sulfur-containing molecules in fossil fuels to sulfone or sulfoxide, and then extracting them with polar solvents to reduce SO₂ emissions during combustion, preventing acid rain formation. The COK-47 series materials synthesized by Bueken et al. (COK-47L and COK-47S) utilize photosensitive ligands and TiO₆ octahedra to construct 2D structures, with COK-47S having abundant methoxy and open sites providing Lewis acid sites, resulting in high catalytic activity towards thiophene compounds, with a catalytic efficiency approximately 11 times that of COK-47L.
Ye et al. studied different temperature-synthesized MIL-125 variants (Ti-BDC). Materials synthesized at higher temperatures (180°C or 220°C) exhibited rich mesoporous structures and open sites, outperforming defect-free MIL-125 in catalytic performance. Notably, Ti-BDC-180 demonstrated excellent performance in the catalytic ODS reactions of BT, DBT, and 4,6-DMDBT, attributed to its rich mesoporous structure and Ti–OH active sites.
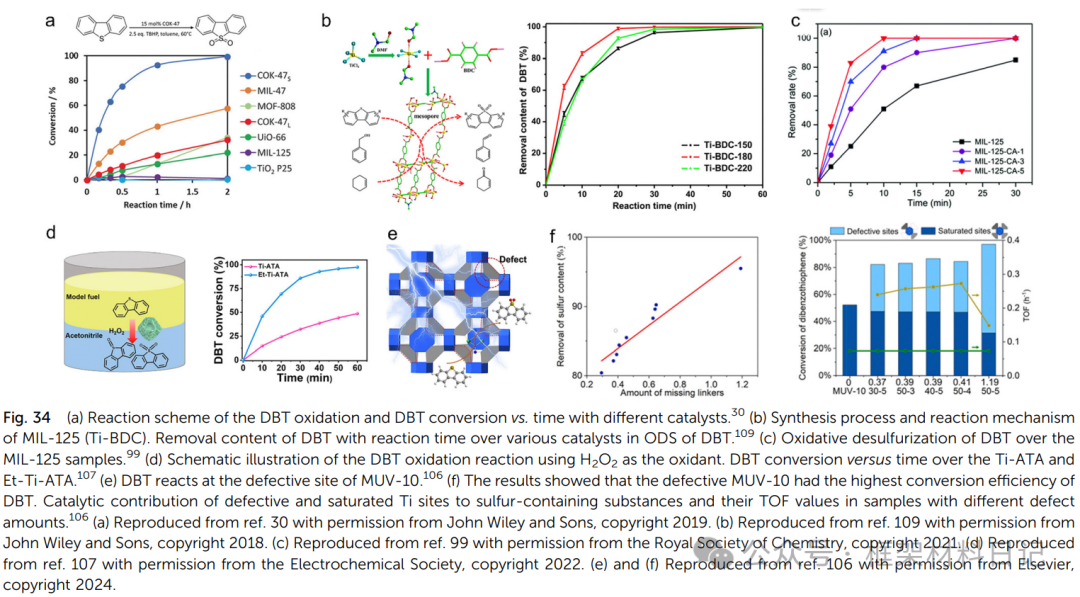
3. Gas Adsorption and Separation
Pore Size and Surface Chemistry Regulation: Defects introduce additional small pores or open metal sites, promoting strong adsorption and selective separation of polar gases (CO₂). The selectivity of defect Ti-MOFs for CO₂/N₂ can be enhanced to >100, outperforming defect-free controls.
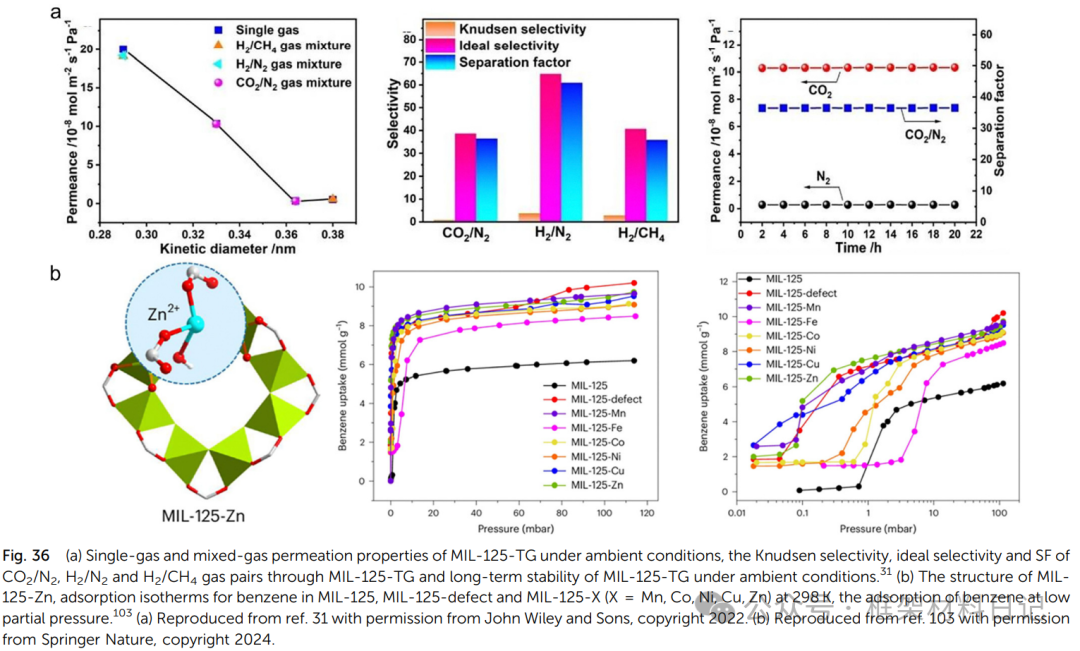
4. Other Functions
Electrocatalysis and Energy Storage: High-density coordination vacancies provide active sites for HER/OER, electrocatalytic CO₂ reduction.
Heterogeneous Catalysis: Open metal sites facilitate the activation of small organic molecules, such as alcohol dehydrogenation and nitro reduction.
Sensing and Drug Delivery: Defect regulation of pore size and hydrophilicity aids in molecular recognition and controlled release.
Conclusion and Outlook
This article reviews the current progress and bottlenecks of defect engineering in Ti-MOFs, looking forward to new strategies through interdisciplinary integration: from molecular design, in situ characterization to intelligent simulation, aiming to achieve an overall transition from “revelation” to “precise construction,” laying the foundation for the industrialization of high-performance Ti-MOFs.
Current Status and Challenges: Ti-MOFs face high synthesis difficulty due to the strong oxygen affinity of Ti⁴⁺ and poor cluster controllability; defect construction must balance structural integrity and functional site exposure.
Future Directions: 1) Develop controllable Ti precursors and new SBUs; 2) Precisely regulate defect location, type, and concentration (multi-scale characterization + in situ monitoring); 3) Synergistic induction of defects with external fields (light, electricity, magnetism); 4) Computational simulations and machine learning to assist defect-performance predictions; 5) Expand to COFs, mixed electronic-ionic transport systems.
Original link:https://doi.org/10.1039/D4CS01007H
Disclaimer:The author acknowledges their limited expertise and welcomes constructive criticism. For any infringement, please contact for resolution.