Vitamin C (hereinafter referred to as VC) is familiar to everyone. Many have heard that supplementing VC can enhance immunity, especially recently due to the threat of the novel coronavirus pneumonia, we all urgently want to improve our immunity to avoid viral infections.

Introduction
VC, also known as ascorbic acid, is an essential water-soluble vitamin for the human body, existing in two active forms: ascorbic acid (AA) and dehydroascorbic acid (DHA). AA can be converted to DHA in the body, which can further hydrolyze into oxalic acid and be excreted in urine, or it can be reduced back to AA in plasma by reduced glutathione for reuse. The history of human understanding of VC can be traced back to ancient China, where descriptions of scurvy-like symptoms can be found in ancient texts. In the 15th to 16th centuries, it was discovered that sailors on long voyages developed a disease characterized by bleeding gums, skin, and mucous membranes, susceptibility to infections, and non-healing wounds, known as “scurvy”. It was later found that this was due to a lack of a nutritional element in their diet, and symptoms improved after supplementing with citrus juice and fresh vegetables. Further research revealed that this element was VC, and humans, due to genetic defects, cannot synthesize VC themselves and must rely on dietary intake. In the 1980s, Dr. Cathcart discovered the relationship between VC and infections, noting that VC levels drop significantly during severe infections, potentially leading to scurvy symptoms. He found that high-dose VC supplementation could significantly improve the prognosis of infections, and as the severity of the infection increased, the tolerance for high-dose VC also gradually increased. He summarized his clinical experience into the “Cathcart VC Intestinal Tolerance Scale”, believing that only when the dosage of VC reaches 80%-90% of the intestinal tolerance can it be effective for acute infections. Moreover, the more severe the disease, the higher the tolerance and demand for VC, even at large doses without causing diarrhea, and this is positively correlated with the severity of the disease. Recent studies have further found that VC also plays a significant role in cardiovascular diseases and cancer treatment.
Sources and Storage of VC
Most vertebrates can synthesize VC in the liver through enzymatic reactions involving glucose, but humans, primates (gorillas, monkeys), fruit-eating bats, guinea pigs, birds, and fish have lost the ability to synthesize VC due to random mutations and the loss of the L-gulono-lactone oxidase (GLO) gene during evolution, thus relying entirely on dietary intake. Our daily food contains abundant VC, with the best sources being fruits and vegetables. However, the growing conditions, ripeness, and storage conditions of food can greatly affect VC content, and VC is very “delicate”, heat-sensitive, easily oxidized, and soluble in water, making it prone to destruction during cooking. Therefore, we must try to consume seasonal, ripe, fresh, and minimally processed vegetables and fruits to ensure adequate VC intake. The human body’s storage pool for VC is very limited, about 1.5g, and when the storage drops below 0.3g (equivalent to a plasma concentration of less than 11uM), scurvy symptoms will appear, necessitating regular and sufficient intake to meet VC needs. A normal human utilizes 40-60mg of VC daily, while stress, pressure, smoking, excessive drinking, and certain disease states may require higher doses. Epidemiological studies indicate that VC deficiency or insufficiency is very common in the population and has been listed as the fourth most common nutritional deficiency in the United States. The normal plasma concentration of VC ranges from 50 to 80μmol/L, but the concentration of VC in tissues is usually 10-100 times higher than that in plasma, reaching millimolar (mmol) levels. It is recommended that normal individuals supplement with 250-300mg of VC daily to maintain physiological needs.

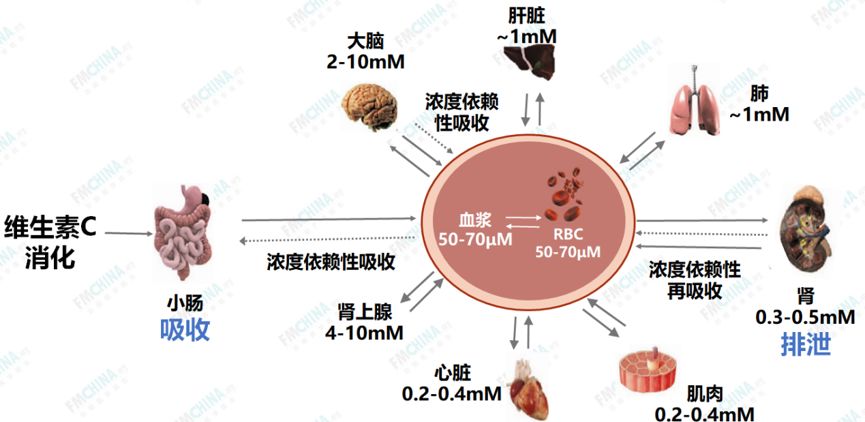 Figure 1: Metabolism, Absorption, and Storage of VC
Figure 1: Metabolism, Absorption, and Storage of VC

Structure and Physicochemical Properties of Vitamin C
In nature, vitamin C generally exists in both reduced and oxidized forms, with the reduced form being L-ascorbic acid and the oxidized form being L-dehydroascorbic acid. They are in dynamic equilibrium and can be converted into each other. The reduced form is the most active and has the strongest effect. However, VC is water-soluble and can easily become inert and oxidized when heated, leading to a decrease in activity. In cooking, for example, excessive heating of vegetables or fruits can lead to a decrease in the VC content of the food, which may affect the nutrient intake from the food. Both L-ascorbic acid and L-dehydroascorbic acid are active and can be interconverted. Vitamin C is generally a white or light yellow powder with a sour taste.
Chemical Structure of Vitamin C
The chemical structure of vitamin C is composed of different carbon, hydrogen, oxygen, and other substances bonded together by various chemical bonds, which can be destroyed by external substances. For example, ultraviolet light can easily damage vitamin C, so vitamin C, along with other nutrients, should not be exposed to direct sunlight and should be stored in the dark. Additionally, vitamin C is acidic and has both reducing and oxidizing forms. It is water-soluble and can easily be oxidized and destroyed during cooking, so it is best to consume seasonal, ripe, fresh, and minimally processed vegetables and fruits. It is well known that vitamin C is acidic and has oxidizing and reducing properties. Therefore, it reacts with different substances. For example, vitamin C can turn purple litmus paper red. Additionally, tannins in tea can bind with trivalent iron to form black tannic iron. VC, being a reducing agent, can reduce trivalent iron in tannic iron to divalent iron, thus causing the tea to lose its black color. This is also a mechanism to identify the action of vitamin C. Iodine solution combined with starch turns blue. VC has a very strong reducing property, which can turn the blue liquid formed by iodine and starch into colorless. Therefore, VC can be seen in vitro to be acidic and has a very strong reducing property, which is its antioxidant function. Vitamin C is affected by various factors, especially functional medicine doctors may supplement VC for patients, particularly those deficient in vitamin C. When supplementing VC, if it is stored for a long time in a high-temperature glass bottle, it may show yellowing and cracking on the surface. We know that the chemical bonds in vitamin C are affected by light, heat, humidity, and metal ions. Therefore, when storing vitamin C, it is essential to avoid these external factors. Vitamin C is sensitive to fire, moisture, heavy metals such as copper and iron, and sunlight. Thus, it is best to store it in brown bottles or bags, sealed, in a cool, dry environment to prevent vitamin C from reducing and oxidizing and deteriorating.
How Oral Vitamin C is Absorbed
For adults and early-stage pregnant women, the recommended daily intake of vitamin C is 100 mg, which is the current recommended dosage in nutrition. For mid to late-stage pregnant women, due to the increased demand for VC by the mother and the fact that breast milk can provide VC to the child, the recommended daily intake increases to 130 mg. Throughout the history of human understanding of vitamin C, it has been found that the demand for vitamin C varies among different populations. In the 1970s, Dr. Cathcart established a personalized assessment method to monitor the VC levels in the body. When oral vitamin C reaches a considerable amount, i.e., 0.5-200 grams within 24 hours, it may be tolerated, but problems may also arise. Therefore, if mild diarrhea occurs when taking oral VC, this dosage is the intestinal tolerance level for VC. Especially when the body is invaded by viruses or bacteria, a large amount of VC is needed. By observing the intestinal tolerance level, personalized dosing can be adjusted for better therapeutic intervention effects. Oral VC is rarely absorbed through the mucosa; absorption occurs mainly in the duodenum and jejunum, where it can be passively absorbed from high to low concentrations, as well as actively transported by intestinal cells to absorb VC into the body. Oral VC is not necessarily fully absorbed by the body. If this portion of VC is not absorbed in the small intestine, it will be transported to the large intestine, where it will be utilized and decomposed by intestinal flora, resulting in gas production. Excessive use of VC without good absorption can lead to waste.
Absorption Rate of VC

The excretion rate of VC can be regulated by the kidneys. When the VC level in the body reaches saturation, urine output increases; if the VC content in tissues is low, urine output decreases. Due to individual differences in intake, the absorption of the intestine, the timing of oral medication, and meal times vary among individuals. It is generally believed that absorption is relatively low on an empty stomach. For example, with 1000 mg of VC, the absorption rate on an empty stomach is 30%, while when taken with food, the absorption rate can reach 50%. Therefore, to avoid wasting VC, it is recommended to take VC after meals, allowing for optimal intake while avoiding side effects. With the continuous development of production technology, fat-soluble VC can be released slowly in the body, allowing for once or twice daily oral dosing.

Physiological Functions of VC


Vitamin C in Hydroxylation Reactions
Hydroxylation reactions are crucial steps in the synthesis and decomposition of bodily substances. Collagen, neurotransmitters, and the detoxification of various drugs all involve hydroxylation reactions. Hydroxylation reactions participate in various physiological functions, such as collagen synthesis, neurotransmitter synthesis, steroid hydroxylation, and the hydroxylation and detoxification of organic drugs and toxins. The hydroxylation of hydroxylysine in collagen requires the activity of prolyl hydroxylase and lysyl hydroxylase, which hydroxylate lysine and proline. VC, as a reducing agent and coenzyme, is fully involved in collagen synthesis. The main components of collagen are hydroxyproline and chondroitin sulfate. When vitamin C is deficient, hydroxyproline and chondroitin sulfate decrease, which can hinder collagen fiber formation and affect connective tissue formation. There is also a large amount of collagen in the vascular wall, and insufficient collagen synthesis can easily lead to various bleeding issues. There are many neurotransmitters in nerve cells and cells, and the conversion of dopamine to norepinephrine requires vitamin C. Once vitamin C is deficient, neurotransmitter synthesis will be hindered, leading to various fatigue and emotional disturbances. Vitamin C promotes steroid hydroxylation, mobilizing cholesterol in the vascular wall to convert into bile acids, reducing cholesterol deposition in the vascular wall and decreasing atherosclerosis. If VC is deficient, atherosclerosis will worsen. Vitamin C can help reverse endothelial dysfunction; when the vascular endothelium is damaged, atherosclerosis begins, and vitamin C can protect low-density lipoprotein cholesterol from free radical attacks. It can also increase high-density lipoprotein, which is a very important mechanism for reversing endothelial damage. Additionally, in families with atherosclerosis, lipoprotein(a) is elevated, and vitamin C can help lower lipoprotein(a) by reacting with insoluble calcium phospholipid cholesterol plaques to convert them into soluble calcium phospholipid cholesterol and ascorbic acid calcium, thus dissolving lipid plaques. Therefore, vitamin C is a nutrient that can reverse endothelial damage. Hydroxylation reactions are related to detoxification; many drugs or toxins require mixed-function oxidases, such as cytochrome P450, to participate in the hydroxylation detoxification process. Vitamin C can enhance enzyme activity, helping to accelerate the metabolism of drugs or toxins.
How Vitamin C Regulates Immunity
To resist viruses and bacteria, the production of antibodies is required. The disulfide bonds in antibodies consist of two cysteines, and high concentrations of vitamin C in the body help convert cystine to cysteine, increasing the levels of various disease-fighting antibodies. A deficiency of vitamin C will lead to a decrease in immunity. Vitamin C increases the activity and function of immune cells, enhances the activity of phagocytes, increases lymphocyte production, and can also enhance the production of interferon, regulating prostaglandin levels, while also having antihistamine and anti-allergic effects. VC can reduce trivalent iron to divalent iron for better absorption. Therefore, when treating iron deficiency anemia, vitamin C is often taken orally alongside iron to facilitate absorption. Glutathione in the body, sulfur-containing compounds, and enzymes containing thiol groups can have their activity increased by vitamin C, enhancing the body’s detoxification functions and various biochemical functions.
Detoxification Function of Vitamin C
Vitamin C is essential for the optimal activity of many important detoxification systems, especially liver cytochrome. The liver cytochrome P450 (CYP450) mixed-function oxidase system and other enzymes responsible for metabolizing toxic substances, including heavy metals (such as mercury, lead, arsenic, cadmium, nickel) and many drugs. It can chelate with toxic heavy metals like lead, mercury, cadmium, and arsenic to form complexes that are excreted from the body. It promotes the reduction of glutathione, where reduced glutathione binds with toxic metals to form complex compounds that are excreted from the body.
Supplementing Adequate Vitamin C Can Prevent Chronic Heavy Metal Poisoning
(1) Anti-cancer function: blocks the formation of N-nitroso compounds, preventing cancer; promotes the synthesis of hyaluronidase inhibitors, stopping cancer cell spread, and reducing the activity of foreign carcinogens in the body. (2) Scavenging free radicals: Vitamin C, NADH2 (reduced hydrogen), and tocopherol have a synergistic effect in scavenging free radicals in the body.
Which Populations Are Prone to Vitamin C Deficiency

Manifestations of VC Deficiency

Adverse Reactions Caused by VC
(1) Diarrhea: High doses of VC can stimulate intestinal peristalsis, causing abdominal pain and diarrhea. For patients with ulcers, such as gastric or duodenal ulcers, VC can increase gastric acid secretion, and its acidic nature can irritate the gastrointestinal mucosa, leading to gastrointestinal bleeding. (2) Stones: VC can be metabolized to form oxalic acid, which is excreted in urine. Long-term high-dose VC use may lead to oxalate stones and gout. Uric acid is a product of purine metabolism, and when uric acid levels are too high, it can cause gout, with gout stones depositing in joints, connective tissues, and kidneys, leading to a series of adverse reactions. Due to the acidic nature of urine, taking high doses of VC is not conducive to uric acid excretion, making gout more likely. (3) Infant dependency syndrome: If pregnant women take large amounts of VC for a long time, the fetus may become dependent on VC. If large doses of VC are not continued after birth, the infant may develop scurvy, including gastrointestinal, skin, and mucous membrane bleeding. Long-term high-dose VC use may lead to poor bone development in children. (4) Allergies: High serum VC levels can reduce the phagocytic ability of white blood cells, thereby lowering immunity. Allergic reactions to VC may manifest as rashes, nausea, vomiting, and even anaphylactic shock.
Intravenous Administration
Intravenous administration results in higher VC concentrations in tissues than oral administration. Intravenous injection of 18 grams/day of vitamin C can produce plasma concentrations 25 times higher than the same dose taken orally. Pharmacokinetic models have confirmed that even with frequent high-dose VC use, plasma concentrations can only slightly increase, from 70 mmol/L to a maximum of 220 mmol/L, while intravenous injection can increase concentrations up to 14,000 mmol/L. The significantly elevated plasma concentration has also been found in vitro to exhibit selective cytotoxicity against tumor cells at plasma concentrations of 1000 to 5000 mmol/L, capable of killing tumor cells. Only intravenous administration can convert VC in tissues into hydrogen peroxide (H2O2), which can be delivered to tumor cells to kill them. The differences between intravenous and oral administration are evident. In summary, functional medicine often treats chronic diseases, and patients with such chronic conditions often use other medications and functional nutrients. The introduction of vitamin C will be shared further, including the interactions between VC and drugs, as well as the interactions between VC and other nutrients. Only by understanding the interconnections between vitamin C, drugs, and diseases can we better apply it clinically.
Instructor Introduction