
Author: Yuanjia Zheng
Editor: Wang Sizhen
The book “Principles of Neurobiology” (Higher Education Press) has become an indispensable treasure trove for researchers and practitioners in neurobiology, authored by Liqun Luo.
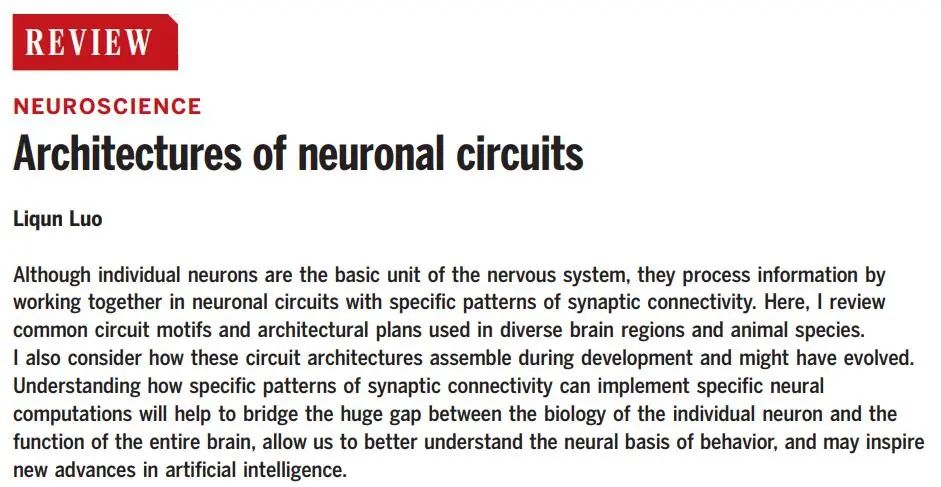
On September 3, 2021, Professor Liqun Luo published a review article titled Architectures of neuronal circuits in Science, summarizing the different connection patterns and functions between neurons studied over the decades using various techniques such as tracing, physiological recording, functional interference, and computational modeling from the perspective of neuronal circuits. The article discusses the potential occurrences of these circuit structures during development and differentiation, providing us with a comprehensive summary of neuronal circuit knowledge.
Introduction
There are approximately 100 billion neurons in the human brain, each with thousands of synaptic connections. Each neuron itself is a complex signal processing unit, but the synaptic connections on neurons allow them to form specialized neuronal circuits, making the brain a truly powerful “computer” system.
As illustrated, if we imagine the brain as an article, then neurons are like the “words” or letters in the article, small local microcircuits are akin to “phrases” or “words”, and large inter-regional neural connections are comparable to “sentences”.
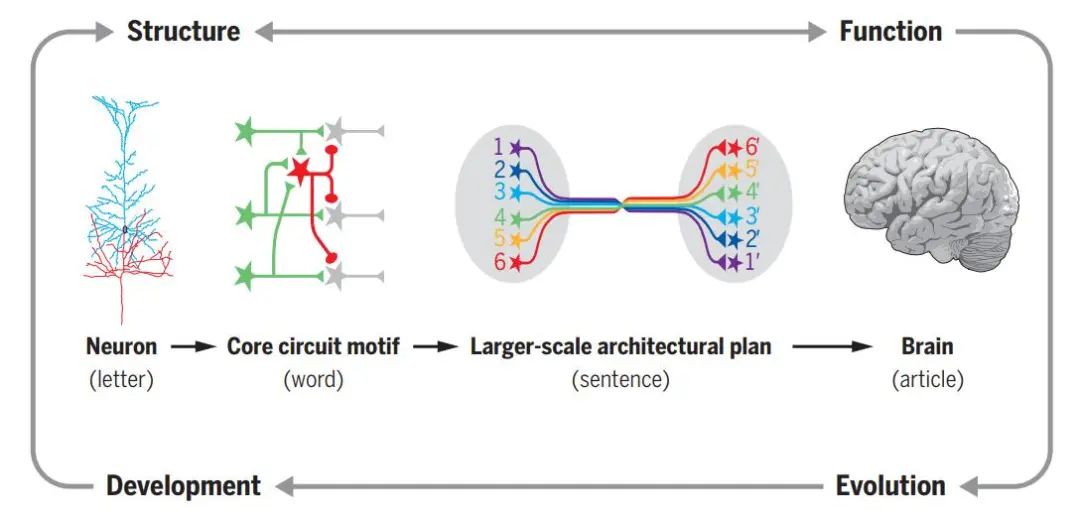
Figure: Neurons and the brain
(Image source: L. Luo, Science 2021; 373: eabg7285)
In the “phrases”, or microcircuit level, specific connections mainly exist between excitatory and inhibitory neurons, forming the most basic information processing functions. These microcircuits are the core units that establish the entire complex signal processing transmission system of the brain. At the “sentence” level, neuronal circuits become more diverse in connections and functions due to anatomical structures and regional expansions. There are numerous recurrent neural circuits in the nervous system, constituting the dynamics of neural activity. Currently, many neuronal circuit structures at this level remain to be discovered.
Although the brain is likened to a computer, the distinction lies in the fact that computers are designed from the top down, while neuronal circuits are products of biological evolution over hundreds of millions of years, not designed by a single “designer”. Some microcircuits in the brain may have originated long ago, preserved during some evolutionary branches, and then evolved into other neural regions. Different neural subsystems have developed independently during evolution, and the proliferation and differentiation of neurons play significant roles in brain evolution, all of which may lead to disruptions in brain connectivity.
Background
Over a century ago, Ramón y Cajal and others proposed that neurons are the basic units of the nervous system, with information transmitted from the dendrites of neurons to the cell body and then to the axon (Figure 1)【1】. However, individual neurons do not work in isolation; they cooperate in neuronal circuits to process information. Therefore, understanding how these connection patterns achieve specific computations will enable us to decipher the principles of information processing in the nervous system and promote advancements in artificial intelligence.
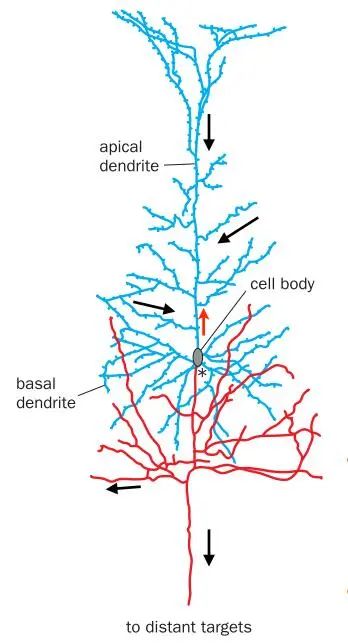
Figure 1: Information transmission in vertebrate neurons
(Image source: L. Luo, Science 2021; 373: eabg7285)
Development
Common circuit motifs—the “words” in the brain’s “article”
1. Feedforward excitation
Feedforward excitation consists of a series of consecutive connections between excitatory neurons, serving as the primary means of signal transmission from one neural region to another (Figure 2A). At each stage, neurons typically receive signal inputs from multiple presynaptic connections (convergent excitation, Figure 2A B) and output signals through branching axons to multiple postsynaptic connections (divergent excitation, Figure 2A C). Convergent excitation allows postsynaptic connections to selectively respond to specific presynaptic neurons. When multiple input neurons carry the same but unrelated signals, it can also enhance the signal-to-noise ratio. Divergent excitation processes the same signal through multiple downstream pathways. For example, in the mammalian visual system, signals flow from photoreceptors → bipolar cells → retinal ganglion cells → lateral geniculate nucleus (LGN) neurons → layer 4 primary visual cortex (V1) neurons → other layers of V1 neurons → cortical upper layer neurons【1-3】. Along these feedforward excitation pathways, visual information transforms from light intensity to contrast, edges, objects, and motion. This feedforward excitation structure in the visual system has inspired the development of “perceptrons” (i.e., graphic recognition machines simulating human visual control systems) and “deep neural networks” for cognition and classification, which artificial intelligence also employs to solve problems beyond image analysis【4】.
2. Feedforward and feedback inhibition
While long-distance signals in the nervous system are primarily transmitted by excitatory neurons (with some exceptions, such as in the basal ganglia and cerebellar circuits), inhibitory interneurons play a crucial role locally【5-6】. Two widely used patterns are feedforward inhibition and feedback inhibition (Figure 2B). In feedforward inhibition, inhibitory neurons receive signal inputs from presynaptic excitatory neurons, thus both inhibitory (Figure 2B C neurons) and presynaptic excitatory (Figure 2B A neurons) inputs converge on the postsynaptic neuron. In feedback inhibition, inhibitory neurons receive inputs from excitatory neurons and project back to the excitatory neurons. In the aforementioned visual pathway, nearly every excitatory connection is accompanied by feedforward inhibition, feedback inhibition, or both. For instance, LGN neurons directly activate V1 GABA-releasing neurons, providing feedforward inhibition to layer 4 excitatory neurons, which also activate V1 GABA-releasing neurons, providing feedback inhibition to themselves【7-8】.
Feedforward inhibition acts faster than feedback inhibition because feedforward inhibition reaches the postsynaptic target cell through only one synapse after the excitatory signal, while feedback inhibition requires two synapses (Figure 2B). Feedforward inhibition is proportional to input strength, while feedback inhibition is proportional to output strength; both are used to regulate the duration and amplitude of incoming excitatory signals. For example, limiting the activation duration in response to sensory input allows circuits to quickly return to their baseline activity levels, maximizing their sensitivity to future environmental changes. Feedforward and feedback inhibition neuronal networks often work in concert to perform many interesting functions, such as regulating the gain and dynamic range of input signals, promoting synchronous or oscillatory firing【6,9】. Feedforward and feedback inhibition also play crucial roles in maintaining the “balance” between excitation and inhibition (e.g., strong excitation accompanied by strong inhibition) to prevent excessive excitation or inhibition states.
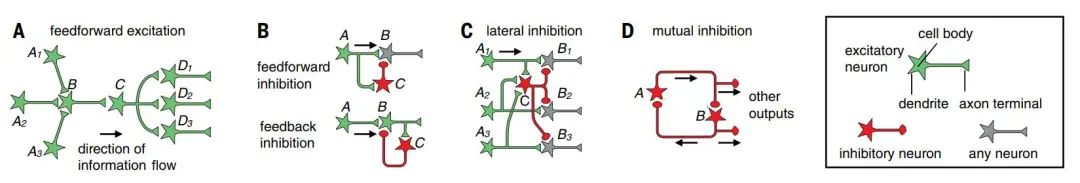
Figure 2: Common circuit motifs. A: Feedforward excitation; B: Feedforward inhibition and feedback inhibition; C: Lateral inhibition; D: Mutual inhibition
(Image source: L. Luo, Science 2021; 373: eabg7285)
3. Lateral inhibition
Lateral inhibition (Figure 2C) is a widely existing circuit motif. It amplifies the activity differences between parallel pathways to select the information to be transmitted to downstream circuits. For example, in the vertebrate retina, photoreceptor neurons activate horizontal cells, providing feedback inhibition to many nearby photoreceptor neurons. This action affects the typical center-surround receptive fields of downstream ganglion cells, enhancing these downstream neurons’ ability to extract spatial or color contrast information【10, 11].
4. Mutual inhibition
The communication between inhibitory neurons can endow circuits with more characteristics. For instance, if inhibitory neuron A directly inhibits inhibitory neuron B, then activation of A will relieve B’s inhibition on target neurons. If B also inhibits A, they form a mutual inhibition pattern (Figure 2D). Mutual inhibition is widely applied in circuits with rhythmic activities, such as those involved in movement【12】. Over longer time scales, mutual inhibition can also be used to regulate brain states, such as the sleep-wake cycle (sleep–wake cycle)【13,14].
The above discussion only involves “words” composed of two “letters”: excitatory neurons and inhibitory neurons. In reality, neuronal microcircuits are very rich. Excitatory and inhibitory neurons exhibit many differences due to their dendritic morphology, ion channel properties, potential characteristics, firing properties, and the subcellular distribution and strength of input and output synapses. For example, in the mammalian neocortex, there are three types of inhibitory neurons: Martinotti cells, basket cells, and chandelier cells, whose presynaptic terminals point to the distal dendrites, cell bodies, and initial segments of excitatory pyramidal neurons, respectively, controlling how pyramidal neurons integrate synaptic inputs and generate spike waves.
In the stomatogastric ganglion, mutually inhibitory neurons have different ion channels and input-output synaptic strengths, which are the basis for their continuous firing within each rhythmic cycle. Additionally, neuronal microcircuits also include many types of modulatory neurons that will be discussed next.
Part Two: Special Circuit Structures with Specific Functions—the “sentences” in the brain’s “article”
The circuit levels to be discussed next are more diverse in scale and configuration and are less easily summarized. The author attempts to summarize some higher-order circuit structure patterns that have been found in multiple neural regions and different species.
1. Continuous topographic mapping
Continuous topographic mapping is a common organizational method for presenting information in the nervous system. Adjacent input neurons project orderly connections to adjacent target neurons through their axons (Figure 3 A). For example, in retinal topographic mapping, adjacent retinal ganglion cells synaptically connect to adjacent LGN neurons, which connect to adjacent V1 neurons, and V1 neurons connect to adjacent higher-order visual cortical neurons. Retinal topographic mapping allows the spatial relationships of the external world captured by the retina to be reproduced in V1 and higher visual cortical areas. In sensory and motor models, sensory stimuli from adjacent body parts are roughly represented in adjacent areas of the primary somatosensory cortex, while motor outputs to adjacent body parts are also controlled by adjacent motor cortical areas.
Due to its robust developmental mechanisms, topographic mapping provides a convenient way to process continuous hierarchical information. It has many computational advantages in information processing. For instance, retinal topographic mapping enhances local contrast extraction capabilities through lateral inhibition, thereby strengthening object recognition. Moreover, by placing circuit elements in adjacent locations, mapping can save energy by minimizing circuit lengths. The design of “convolutional neural networks” (which include convolutional computations and have deep structures, representing one of the algorithms of deep learning) draws on topographic mapping, significantly reducing the number of variables needed to adjust artificial neural networks, thus accelerating computation speed【4, 15].
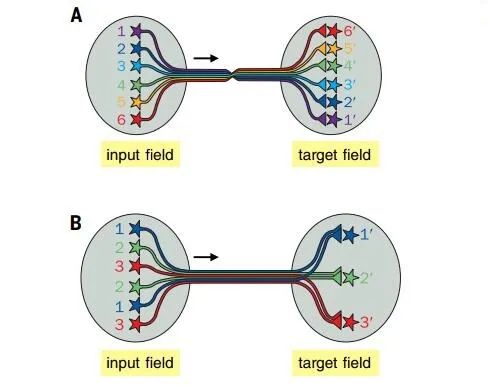
Figure 3: Specific circuit structures for specific functions. A: Continuous brain map; B: Discrete parallel processing.
(Image source: L. Luo, Science 2021; 373: eabg7285)
2. Discrete parallel processing
Discrete parallel processing (Figure 3B) presents and processes signals in parallel through discrete information channels. A typical example is the vascular glomeruli organization in vertebrate olfactory bulbs and insect antennal lobes: olfactory receptor neurons expressing the same odorant receptors send their axons to the same glomeruli, which then synaptically connect to the dendrites of their corresponding secondary projection neurons, forming discrete olfactory processing channels【16,17】. Different axons aggregating to the same glomerulus enhance the signal-to-noise ratio. Different glomeruli represent not continuous signals but discrete signals from olfactory receptor neurons, reflecting the nature of the chemicals activating these odorant receptors. Discrete parallel processing is also a feature of the mammalian gustatory system.
Discrete parallel processing is often used in conjunction with continuous topographic mapping. For instance, in the retina, superimposed on the retinal topographic mapping are discrete different layers, where different types of bipolar and ganglion cells form specific connections and process different types of visual signals in parallel, such as brightness, color, and motion. Compared to serial processing of information, parallel processing reduces computational depth, thereby lowering error rates and increasing processing speed. A significant feature of complex nervous systems (with a large number of neurons, each with numerous connections) is their ability to perform large-scale parallel processing, a structure increasingly used in the design of computer systems【18,19].
3. Dimensionality expansion
In dimensionality expansion structures, signals from a relatively small number of input neurons diverge to a much larger number of output neurons (Figure 3 C), allowing output neurons to present different combinations of signal inputs. For example, in insects’ mushroom bodies (olfactory projection neurons → mushroom body Kenyon cells → mushroom body output neurons) and vertebrates’ cerebellum (mossy fibers → cerebellar granule cells → Purkinje cells). In both cases, a relatively small number of input neurons (projection neurons or mossy fibers) synaptically connect to a large number of output neurons (Kenyon cells or granule cells). Thus, the information at the output neuron level can be presented in a higher-dimensional space, with each dimension representing a cell’s firing rate. The firing patterns of postsynaptic neurons can be used to distinguish subtle differences in input patterns (e.g., different populations of projection neurons representing different odor combinations). This structure allows learning by adjusting the synaptic strengths of output neurons, so that after training, the same input can produce different output patterns (Figure 3 C).
Another example of dimensionality expansion is the entorhinal cortex → dentate gyrus granule cells → CA3 pyramidal circuit (Figure 3 D above). A large number of dentate gyrus granule cells can perform pattern separation of spatial and object information from the entorhinal cortex, which is further processed by downstream hippocampal circuits【20,21】. However, unlike the mushroom body and cerebellar cortex, this circuit cannot perform learning and training. This may be because the hippocampal microcircuits engage in unsupervised learning, while the cerebellar and mushroom body microcircuits engage in supervised reinforcement learning.
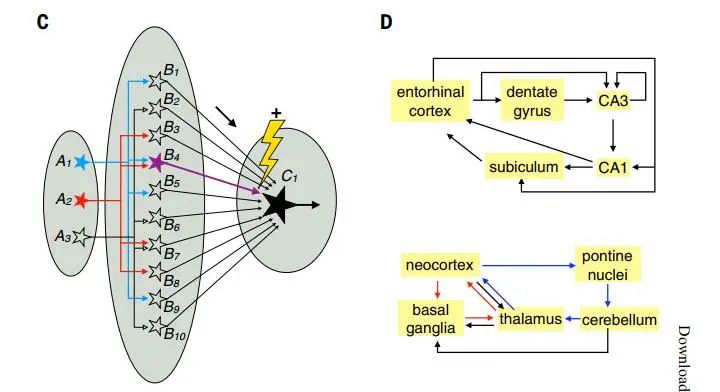
Figure 3: Specific circuit structures for specific functions. C: Dimensionality expansion; D: Recurrent circuits at the level of mammalian brain neuron populations (top) and at the level of mammalian brain regions (bottom).
(Image source: L. Luo, Science 2021; 373: eabg7285)
4. Recurrent loops
The nervous system is filled with recurrent loops, in which neurons are typically interconnected through interneurons. These recurrent loops are uneven in scale, spanning from specific neural regions to most areas of the brain. For example, in the mammalian visual system, in addition to the “bottom-up” projections from LGN → V1 → higher cortical areas, the “top-down” projections from higher cortical areas → V1 → LGN also serve various functions, including attention control. Long-distance recurrent loops can contain continuous topographic mapping or discrete parallel processing architectures (Figure 3 D). Recurrent loops typically support rich neural activity, but in most cases, their exact roles remain unclear and may vary depending on specific circumstances. Understanding the principles and functions of information processing in recurrent loops is also a major challenge in modern neuroscience.
5. Biased input–segregated output
In addition to circuits composed of excitatory and inhibitory neurons, the nervous system also utilizes modulatory neurons to perform important functions. Since modulatory neurons transmit signals through neurotransmitters (such as monoamines and neuropeptides), their effects on postsynaptic neurons are slower compared to fast excitatory and inhibitory neurotransmitters (which bind to ionotropic receptors). Besides acting through synaptic gaps, modulatory neurotransmitters can also be released at non-specific sites on postsynaptic neurons, known as “volume release”, allowing them to affect targets over greater distances than typical synaptic gaps.
Using mouse viral tracing techniques, it has been observed that the midbrain dopamine, ventral serotonin, and hypothalamic neuropeptide systems adopt a “biased input–segregated output” structure (Figure 4 A)【22,23】. Different target areas for different behavioral functions are segregated in their outputs, with each system divided into multiple parallel subsystems. Each output subsystem receives a certain amount of biased input from similar areas, allowing these subsystems to be modulated differently by external and internal stimuli. However, in another case, such as the locus coeruleus norepinephrine system, although the branching patterns of individual neurons may be specific, their axons project to one brain area while also broadly projecting to other areas. These observations suggest that the locus coeruleus norepinephrine system adopts an integrative transmission architecture (Figure 4 B), which may suit its role in modulating the overall state of the brain (e.g., arousal).
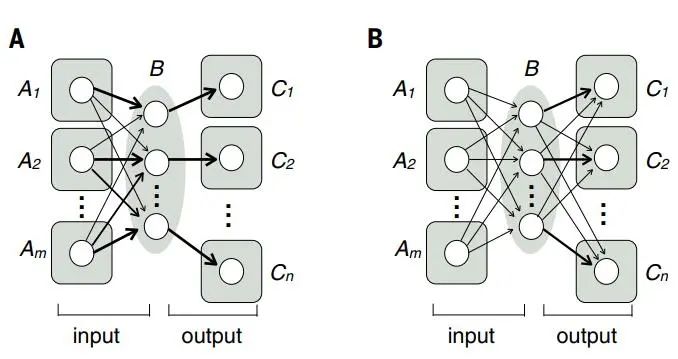
Figure 4: Input-output of neural modulation circuits. A: Biased input–segregated output structure; B: Integrative–propagative structure.
(Image source: L. Luo, Science 2021; 373: eabg7285)
In addition to the circuit architecture patterns summarized above, other architectures have also been discovered, such as the mammalian neocortex and glial circuits, which do not fully conform to the above categories. Future exploration of the depth and breadth of neuronal circuits is still needed. Only when we know more about the “sentences” and understand the changes and complex interactions within them can we gain deeper insights into their composition (such as “paragraphs”) and even the entire “article”, that is, the entire nervous system.
Part Three: Perspectives on Evolution and Development
Computer circuits are products designed from the top down, while complex neuronal circuits have evolved over hundreds of millions of years. Neuronal circuits also utilize evolutionary selection and experience for self-assembly or fine-tuning during development. Observing a single neuronal circuit may not reveal which elements are functionally important, but observing what has been selected, expanded, reduced, eliminated, or redundantly produced during evolution can suggest which elements functional studies should focus on.
1. Evolution of neuronal circuits
The existing bilaterally symmetrical nervous system may have gradually evolved and matured; first, there were only muscle cell neurons, followed by a series of evolutionary developments of sensory-motor neurons, from independent sensory and motor neurons to interneurons, central neural networks, ultimately leading to the emergence of the central nervous system and brain【24, 25】. Some core patterns, such as feedforward excitation and feedforward/feedback inhibition, may have begun early in animals with interneurons and central nervous systems, as their effects have preserved these patterns across different species and transmitted them in various neural regions of each species. Other architectures have also evolved independently, such as the vascular glomeruli organization in the olfactory systems of insects and vertebrates, likely resulting from convergent evolution, as other biological branches evolved from their common ancestors do not possess such organization and utilize different types of molecules as odorant receptors.
The gradual complexity of the nervous system requires an increase in the number of neurons, types of neurons, and the expansion of their connections and brain regions. All these processes are driven by changes in DNA. A key mechanism of evolutionary innovation is gene duplication and differentiation; for example, the duplication and differentiation of cone opsin genes have enabled certain primates to have trichromatic vision【26】. Duplication and differentiation are also used for the evolution of neuron types and brain regions【27, 28】. In principle, the repeated and divergent evolution of brain regions can modularize neuronal circuit structures, allowing for rich connections within repeated units and sparse connections between units. In turn, the modular characteristics of neuronal circuits may accelerate evolution, as different modules can evolve independently.
2. Development of neuronal circuits
Evolution primarily modifies the genes involved in circuit connections during development, affecting neuronal circuits. A critical question is how a limited number of genes can construct a vast number of synaptic connections with specific patterns and structures in the nervous system. Extracellular signals and their cell surface receptors can recognize specific targets through axonal and dendritic growth cones, serving as the main molecules for establishing the initial organization of the nervous system, which can also specify synaptic connections very precisely in certain circuits and organisms【29, 30】.
To establish a large number of connections with specificity using a limited number of genes, one strategy is to use different expression levels of the same protein to specify different connections. This strategy can be used to construct continuous topographic mappings (Figure 5 A), which may also be the reason for the richness of this circuit structure. Gradients of cell surface molecules are also used to construct early discrete mappings. However, discrete parallel processing requires distinguishing discrete cell types and often employs combinatorial cell surface protein coding, allowing a small number of proteins to specify more connections (Figure 5 B left). The method of achieving combinatorial coding is to divide the connection process into different spatiotemporal steps (Figure 5 B right). Besides saving molecules, this strategy can also enhance stability. Through complex spatiotemporal regulation of their expression patterns, the same connection molecules can be used at different times and places or in different parts of the same circuit【31, 32】.
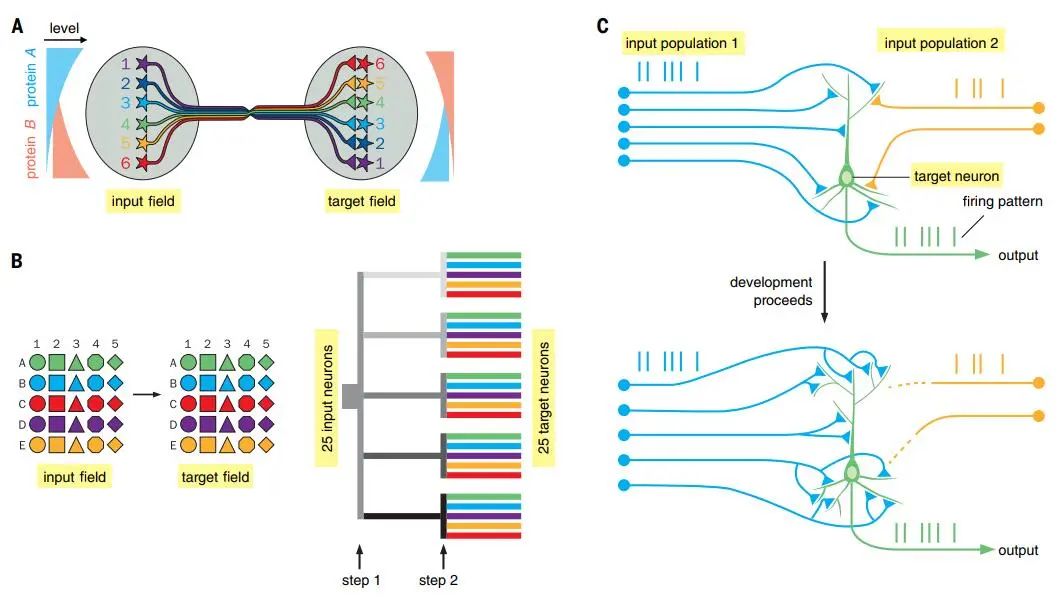
Figure 5: Connecting neuronal circuits. A: Protein gradients construct continuous topographic mappings; B: Combinatorial strategies construct specific connections; C: Hebbian law constructs specific connections.
(Image source: L. Luo, Science 2021; 373: eabg7285)
Spontaneous and experience-driven neuronal activity also refines synaptic connections. Activity-dependent synaptic connections have been shown to arise from competition between neurons with different activity levels. An important mechanism by which neuronal activity influences connections is through Hebb’s rule: the firing of presynaptic neurons strengthens the firing of postsynaptic neurons, i.e., “cells that fire together, wire together” (Figure 5 C). Additionally, non-Hebbian mechanisms, such as homeostatic synaptic plasticity, also contribute to activity-dependent circuit connections【33】, which persist in the adult nervous system, allowing animals to modify their synaptic connection patterns based on their life experiences.
However, many synaptic connections are not entirely specific. For instance, in the vertebrate neuromuscular system, the connections between motor neuron pools and muscles are specific, but the specific connection patterns between motor neurons and muscle fibers are highly variable【34】. Similarly, in the olfactory circuits of fruit flies, the synaptic connections between specific olfactory projection neuron types and mushroom body Kenyon cells are mostly random【35, 36】.
In summary, there are two common mechanisms for establishing connection patterns in neuronal circuits: 1. Molecular connections in the nervous system, and 2. Neuronal activity adjusting connections based on experiences. There is also interaction between neuronal activity and molecular connections; for example, neuronal activity can regulate the expression of molecular connections or complement the effects of molecular connections【37, 38】. However, aside from the few examples discussed above, most developmental studies have yet to address how circuit patterns and architectures are established, and most studies on circuit functions have not considered developmental limitations. Therefore, cross-research on the development and function of neuronal circuits will help answer these questions.
Conclusion and Outlook
The application of circuit research tools, such as electron microscopy and trans-synaptic tracing in different organisms and neural regions, can yield a wealth of data to explore the common laws of neuronal circuit architecture. Recording neuronal activity and interfering with key factors in circuits can help understand their functions in information processing and animal behavior. The author believes that a key challenge in the future is how different circuit patterns and structures operate across scales. In the critical circuit structures across species, studying how “words” and “phrases” combine into “sentences” is more valuable, and using single-cell transcriptomics to compare different neuronal types in homologous brain regions is also a feasible method to initiate related research. A comprehensive study of the structure, function, development, and evolution of neuronal circuits will enable us to move beyond the level of individual neurons and gain deeper insights into the nervous system, which will also inspire new artificial neural networks, potentially achieving general artificial intelligence one day.
Original link:https://doi.org/10.1126/science.abg7285
Selected Previous Articles【1】EMBO J: New discovery! AGHGAP11B promotes neocortex expansion into adulthood and enhances cognitive abilities.
【2】Cell Death Differ: Qi Yitao/Wu Hongmei and others reveal the molecular mechanisms regulating adult neurogenesis through SUMOylation modifications.
【3】Cereb Cortex: A2A receptor antagonists can reverse sequence learning deficits induced by abnormal aggregation of α-Syn.
【4】Neuron: New findings on nicotine promoting anxiety—important role of inhibiting the ventral tegmental area-amygdala dopamine pathway.
【5】Int J Mol Sci: Frontier review interpretation: Pathophysiological responses and roles of astrocytes in traumatic brain injury.
【6】Cereb Cortex: Wang Lang’s research group reveals experience-dependent homeostatic plasticity in astrocytes.
【7】Nature: New discovery! Oxytocin neurons induce social transmission of maternal behavior.
【8】Genome Biol: Ding Junjun’s team systematically maps the three-dimensional chromatin structure of phase separation dissolution and reconstruction processes.
【9】Brain: New method! Plasma astrocyte proliferation GFAP may be a potential biomarker for Alzheimer’s disease detection.
【10】Autophagy: Zhang Zhidong’s team reveals a new mechanism of STING1-induced autophagy regulation of RNA virus infection.
【11】Nature: Astrocyte-derived IL-3 regulates microglial function, alleviating AD pathological changes and cognitive impairment.
【12】JCI: Gao Tianming’s research group reveals opposing roles of neural circuits in the prefrontal cortex in regulating anxiety and fear.
【13】eLife: Single-cell sequencing and neural circuit analysis jointly reveal the molecular genetic coding mechanisms of the brain’s attack/defense instinct behaviors.
【14】Nature: Cutting-edge! GluDs transmit different presynaptic signals to different postsynaptic receptor responses.

References (Scroll up and down to view)
【1】 S. R. Cajal, Histology of the Nervous System of Man and Vertebrates (Oxford Univ. Press, 1995).
【2】 C. D. Gilbert, T. N. Wiesel, Morphology and intracortical projections of functionally characterised neurones in the cat visual cortex. Nature 280, 120–125 (1979).
【3】 D. J. Felleman, D. C. Van Essen, Distributed hierarchical processing in the primate cerebral cortex. Cereb. Cortex 1, 1–47 (1991).
【4】 Y. LeCun, Y. Bengio, G. Hinton, Deep learning. Nature 521, 436–444 (2015).
【5】 C. S. Sherrington, Remarks on some aspects of reflex inhibition. Proc. R. Soc. London Ser. B 97, 519–545 (1925).
【6】 J. S. Isaacson, M. Scanziani, How inhibition shapes cortical activity. Neuron 72, 231–243 (2011).
【7】 K. A. Martin, P. Somogyi, D. Whitteridge, Physiological and morphological properties of identified basket cells in the cat’s visual cortex. Exp. Brain Res. 50, 193–200 (1983).
【8】 X. Y. Jie et al., Thalamocortical innervation pattern in mouse auditory and visual cortex: Laminar and cell-type specificity. Cereb. Cortex 26, 2612–2625 (2016).
【9】 L. Roux, G. Buzsáki, Tasks for inhibitory interneurons in intact brain circuits. Neuropharmacology 88, 10–23 (2015).
【10】 S. W. Kuffler, Discharge patterns and functional organization of mammalian retina. J. Neurophysiol. 16, 37–68 (1953).
【11】 H. B. Barlow, Summation and inhibition in the frog’s retina. J. Physiol. 119, 69–88 (1953).
【12】 S. Grillner, Biological pattern generation: The cellular and computational logic of networks in motion. Neuron 52, 751–766 (2006).
【13】 C. B. Saper, P. M. Fuller, N. P. Pedersen, J. Lu, T. E. Scammell, Sleep state switching. Neuron 68, 1023–1042 (2010).
【14】 F. Weber, Y. Dan, Circuit-based interrogation of sleep control. Nature 538, 51–59 (2016)
【15】 I. E. Wang, T. R. Clandinin, The influence of wiring economy on nervous system evolution. Curr. Biol. 26, R1101–R1108 (2016).
【16】 R. Axel, The molecular logic of smell. Sci. Am. 273, 154–159 (1995).
【17】 L. B. Vosshall, R. F. Stocker, Molecular architecture of smell and taste in Drosophila. Annu. Rev. Neurosci. 30, 505–533 (2007).
【18】 L. Luo, Principles of Neurobiology (CRC Press/Garland Science, ed. 2, 2020).
【19】 A. Krizhevsky, I. Sutskever, G. Hinton, Imagenet classification with deep convolutional neural networks. In Proc. Adv. Neural Inf. Process. Syst. 25, 1090–1098 (2012).
【20】 D. Marr, Simple memory: A theory for archicortex. Philos. Trans. R. Soc. Lond. B Biol. Sci. 262, 23–81 (1971)
【21】 J. P. Neunuebel, J. J. Knierim, CA3 retrieves coherent representations from degraded input: Direct evidence for CA3 pattern completion and dentate gyrus pattern separation. Neuron 81, 416–427 (2014)
【22】 K. T. Beier et al., Circuit architecture of VTA dopamine neurons revealed by systematic input-output mapping. Cell 162, 622–634 (2015).
【23】 J. Kohl et al., Functional circuit architecture underlying parental behaviour. Nature 556, 326–331 (2018).
【24】 L. W. Swanson, Brain Architecture (Oxford Univ. Press, ed. 2, 2012).
【25】 J. H. Kaas, Evolutionary Neuroscience (Elsevier, ed. 2, 2020).
【26】 G. H. Jacobs, J. Nathans, The evolution of Primate color vision. Sci. Am. 300, 56–63 (2009)
【27】 M. A. Tosches et al., Evolution of pallium, hippocampus, and cortical cell types revealed by single-cell transcriptomics in reptiles. Science 360, 881–888 (2018).
【28】 R. D. Hodge et al., Conserved cell types with divergent features in human versus mouse cortex. Nature 573, 61–68 (2019).
【29】 A. L. Kolodkin, M. Tessier-Lavigne, Mechanisms and molecules of neuronal wiring: A primer. Cold Spring Harb. Perspect. Biol. 3, a001727 (2011)
【30】 J. R. Sanes, S. L. Zipursky, Synaptic specificity, recognition molecules, and assembly of neural circuits. Cell 181, 536–556 (2020).
【31】 W. J. Joo, L. B. Sweeney, L. Liang, L. Luo, Linking cell fate, trajectory choice, and target selection: Genetic analysis of Sema-2b in olfactory axon targeting. Neuron 78, 673–686 (2013)
【32】 D. T. Pedrick et al., Reciprocal repulsions instruct the precise assembly of parallel hippocampal networks. Science 372, 1068–1073 (2021).
【33】 G. G. Turrigiano, The dialectic of Hebb and homeostasis. Philos. Trans. R. Soc. London Ser. B 372, 20160258 (2017).
【34】 J. Lu, J. C. Tapia, O. L. White, J. W. Lichtman, The interscutularis muscle connectome. PLOS Biol. 7, e32 (2009).
【35】 S. J. Caron, V. Ruta, L. F. Abbott, R. Axel, Random convergence of olfactory inputs in the Drosophila mushroom body. Nature 497, 113–117 (2013).
【36】 Z. Zheng et al., A complete electron microscopy volume of the brain of adult Drosophila melanogaster. Cell 174, 730–743.e22 (2018).
【37】 S. Serizawa et al., A neuronal identity code for the odorant receptor-specific and activity-dependent axon sorting. Cell 127, 1057–1069 (2006).
【38】 T. McLaughlin, C. L. Torborg, M. B. Feller, D. D. O’Leary, Retinotopic map refinement requires spontaneous retinal waves during a brief critical period of development. Neuron 40, 1147–1160 (2003).
Typesetting: Wang Sizhen
This article is complete
