
The redox homeostasis of mitochondria is crucial for mitochondrial function and cellular status, achieved through the intricate interplay between oxidative stress factors (such as reactive oxygen species/reactive nitrogen species ROS/RNS) and the antioxidant defense system. An imbalance in redox state can lead to protein damage, ultimately resulting in tissue injury. For instance, methionine residues in proteins are particularly susceptible to oxidation byROS, forming methionine sulfoxide (MetSO), which disrupts the structure and function of related proteins. As a key antioxidant enzyme, methionine sulfoxide reductase (Msrs) is currently the only known enzyme that can specifically catalyze the reduction ofMetSO back to methionine (Met), thereby restoring the activity of methionine-containing proteins. Therefore,Msrs is not only critical for protein repair but also plays an irreplaceable role in the antioxidant system through redox cycling. Abnormal fluctuations in the levels ofMsrs in neurons are closely associated with a range of oxidative stress-related neurodegenerative diseases (such as Alzheimer’s diseaseAD, Parkinson’s diseasePD, etc.). Based on this correlation, there is an urgent need to develop sensitive and precise methods for detecting and imaging mitochondria-specificMsrs activity in living neurons.
With its high sensitivity, biocompatibility, and non-destructive detection capabilities, fluorescence sensing technology (especially dual-channel ratiometric imaging) is considered an ideal approach for in situ studies of biomolecules (including enzymes). Compared to traditional fluorescence intensity imaging, fluorescence lifetime imaging (FLIM) offers superior temporal resolution for precise identification of luminescent signals, even when their emission spectra overlap but decay kinetics differ, making it a more promising biological imaging technology (with better precision and accuracy). Its key advantage lies in the fact that luminescence lifetime is highly insensitive to changes in intracellular probe concentration or fluctuations in excitation laser power, and lifetime signal analysis can effectively alleviate irregular and unpredictable signal decay caused by tissue component heterogeneity (a challenge inherent in intensity-based detection methods). However, it should be noted that despite the advantages ofFLIM, there are still numerous endogenous luminescent substances in biological systems (whose fluorescence decay rates are on the same order of magnitude as traditional fluorescent probes, approximately in the nanosecond range), which produce unavoidable background interference, limiting the accuracy of lifetime sensing. Therefore, how to further reduce interference and improve detection accuracy in live cell analysis remains a significant challenge.
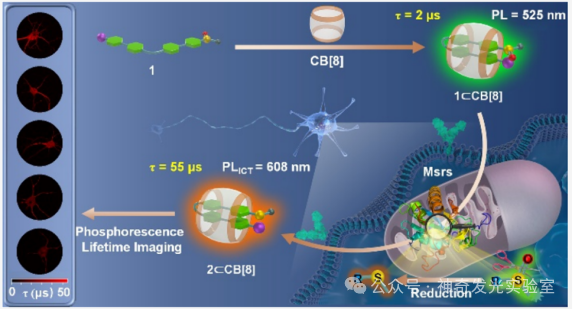
Based on the authors’ previous research on the regulation of photophysical and biosensing properties through supramolecular host-guest interactions, this paper designs and synthesizes a novel supramolecular probe (1⊂CB). This probe exhibits microsecond room-temperature phosphorescence (RTP) properties in aqueous solution and enables high-sensitivity and high-precision detection of mitochondria-targetedMsrs activity through dual-channel phosphorescence ratiometry and phosphorescence lifetime imaging (PLIM). When the probe is exposed toMsrs, its sulfoxide group is reduced to a stronger electron-donating thioether group, forming a new phosphorescent product2⊂CB. This structural transformation prompts the occurrence of a stable intramolecular charge transfer (TS-ICT) process, leading to a significant red shift in phosphorescent emission wavelength (from525 nm to608 nm), extending the long wavelength into the near-infrared (NIR region (800 nm), thus generating a dual-channel ratiometric phosphorescent response toMsrs. Simultaneously, the enzyme-activatedTS-ICT emission mechanism also significantly extends the phosphorescence lifetime from2 μs to55 μs, providing a pathway for accurately quantifyingMsrs levels throughPLIM technology. This strategy effectively eliminates interference from biological autofluorescence that is three orders of magnitude shorter in lifetime. Utilizing the excellent properties of this phosphorescent supramolecular probe, it is further applied to sensing and imagingMsrs levels in living neurons and mouse brain tissues. Notably, in theAD model, the activity ofMsrs in neuronal mitochondria and the CA1 region of the hippocampus is significantly reduced, indicating that the pathology ofAD is accompanied by impaired antioxidant and protein repair functions.

Figure 1: Design Strategy
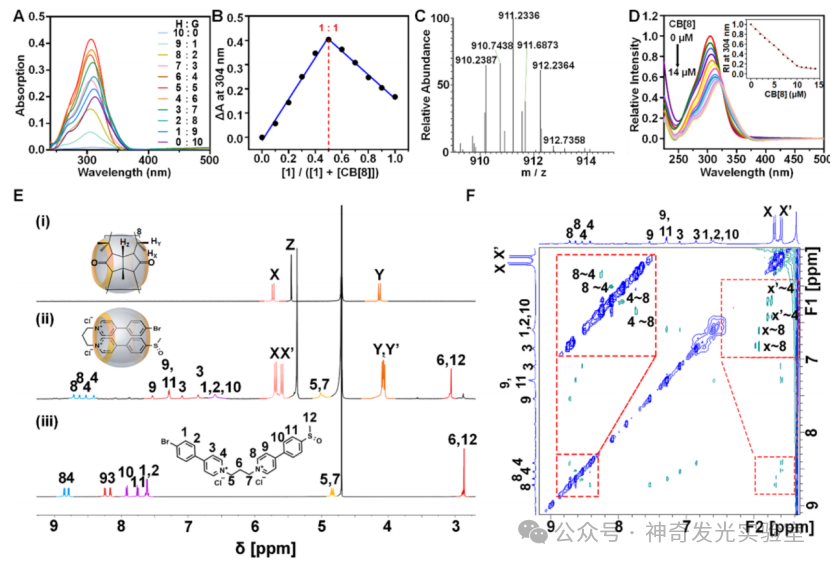
Figure 2: Photophysical Characterization
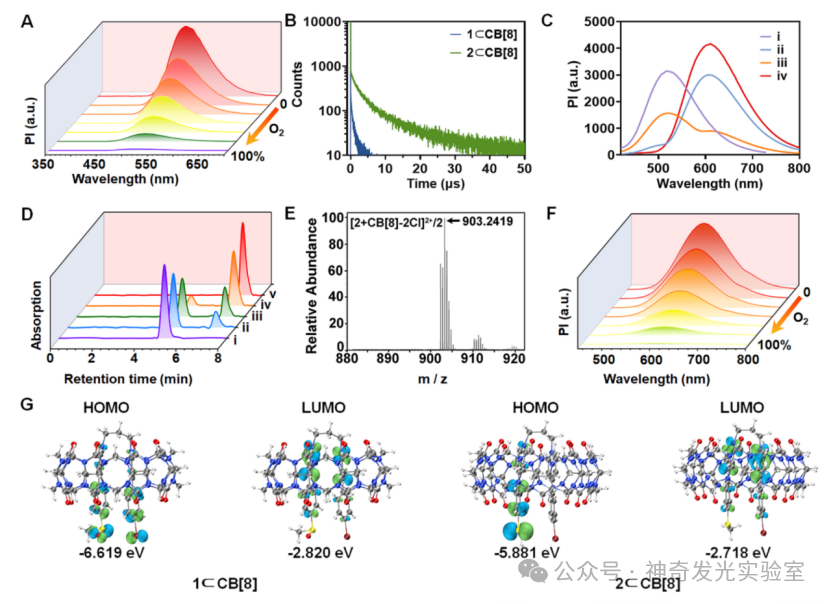
Figure 3: Photophysical Properties and Response Mechanism of Supramolecular Phosphorescent Probes toMsrs.
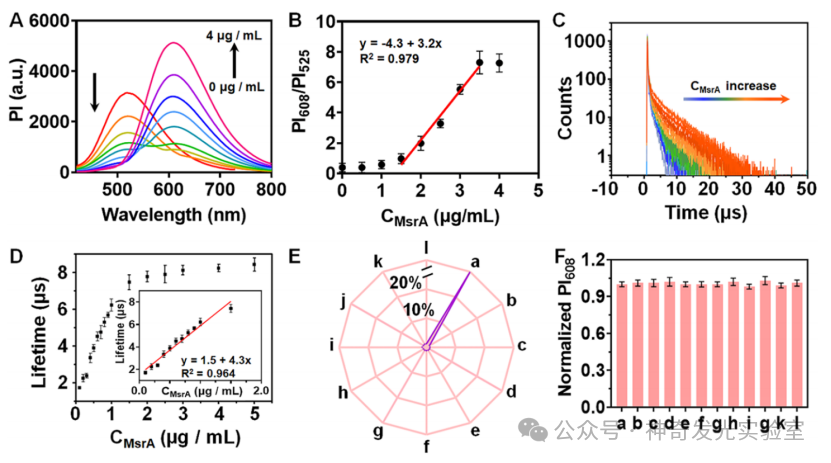
Figure 4: Study of the In Vitro Fluorescent Sensing Performance of Supramolecular Probes forMsrs.
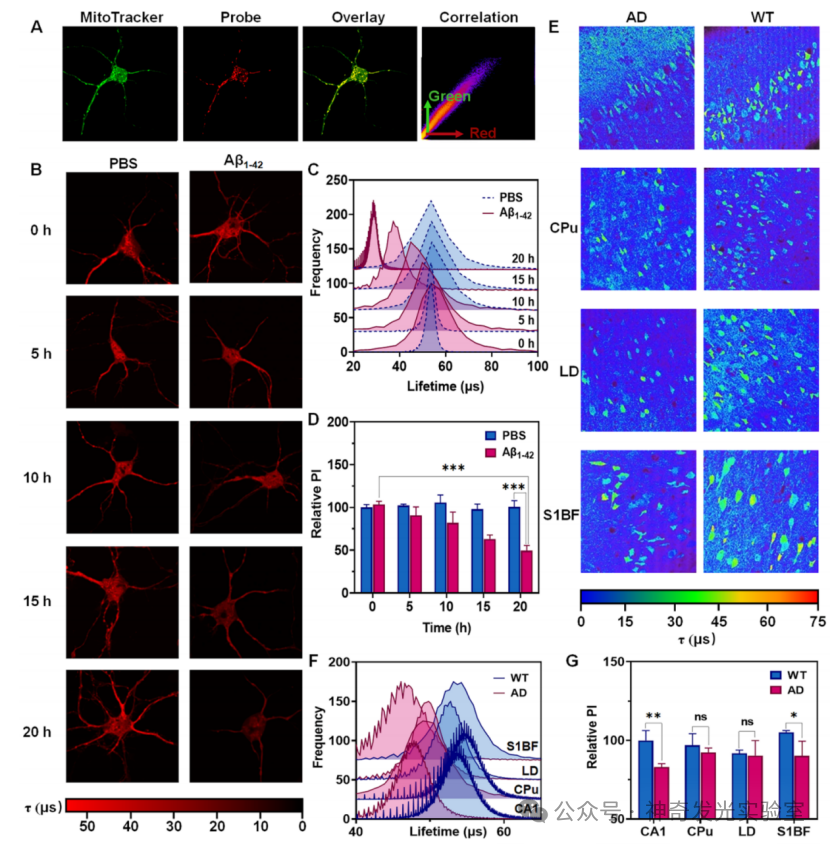
Figure 5: Phosphorescence Lifetime Imaging of Mitochondria-SpecificMsrs Activity in Living Neurons and Acute Brain Slices of AD Model Mice.
[Literature Details]Xuewei Wang, Chen Chen, Yang Tian, and Qi-Wei Zhang. Dual-Channel Phosphorescence Ratiometry and Phosphorescence Lifetime Imaging of Mitochondria-Specific Methionine Sulfoxide Reductase Activity.J. Am. Chem. Soc., 2025, https://doi.org/10.1021/jacs.5c03235