Due to the different reaction mechanisms of the Ammonia Oxidation Reaction (AOR) and Hydrogen Evolution Reaction (HER), there is currently a lack of dual-function catalysts that can effectively catalyze both reactions. In this study,we used NaH2PO2 as the phosphorus source to dope phosphorus (P) into the PtZn alloy through calcination, inducing lattice strain in platinum (Pt) and electronic interactions between phosphorus (P) and zinc (Zn), which optimizes the activity for both AOR and HER.The sample with the optimal phosphorus content achieves a peak current density of 293.6 mA·mgPt−1 in AOR, which is nearly 2.7 times that of pure platinum (Pt). For HER, the overpotential at −10 mA·cm−2 is only 23 mV, with a Tafel slope of 34.1 mV·dec−1. Furthermore, in a two-electrode system, only 0.59 V is required to achieve an ammonia electrolysis current of 50 mA·mgPt−1. Therefore, this study demonstrates a clever design for ammonia electrolysis dual-function catalysts.
 Lack of Dual-Function Catalysts for AOR and HER: Due to the different reaction mechanisms, it is currently difficult to find a catalyst that can effectively catalyze both reactions.
Importance of Catalytic Performance Optimization: Optimizing the electronic properties and structure of catalysts is crucial for enhancing the activity of AOR and HER.
Catalyst Material: PtZn alloy, modified by phosphorus (P) doping.
Preparation Method: Using the polyol method and calcination process, phosphorus is doped into the PtZn alloy with NaH2PO2 as the phosphorus source.
Doping Amount Control: The doping amount of phosphorus can be controlled by adjusting the calcination temperature, which affects the lattice strain and electronic interactions of the catalyst.
Lack of Dual-Function Catalysts for AOR and HER: Due to the different reaction mechanisms, it is currently difficult to find a catalyst that can effectively catalyze both reactions.
Importance of Catalytic Performance Optimization: Optimizing the electronic properties and structure of catalysts is crucial for enhancing the activity of AOR and HER.
Catalyst Material: PtZn alloy, modified by phosphorus (P) doping.
Preparation Method: Using the polyol method and calcination process, phosphorus is doped into the PtZn alloy with NaH2PO2 as the phosphorus source.
Doping Amount Control: The doping amount of phosphorus can be controlled by adjusting the calcination temperature, which affects the lattice strain and electronic interactions of the catalyst.

03 Catalyst Characterization
Physicochemical Characterization: The morphology, structure, and surface chemical information of the catalyst were characterized using techniques such as HRTEM (High-Resolution Transmission Electron Microscopy) and XPS (X-ray Photoelectron Spectroscopy).
Lattice Strain and Electronic Interactions: The doping of phosphorus induces lattice strain in platinum and enhances electronic interactions between phosphorus and zinc, thereby optimizing the activity for AOR and HER.
04 Electrochemical Performance
AOR and HER Activity Testing: The activity of the catalyst in AOR and HER was evaluated through electrochemical testing.
Optimal Doping Amount: When the phosphorus content is 3.80 wt.%, the catalyst exhibits the highest peak current density for AOR (293.6 mA·mgPt−1), indicating optimal performance at this doping level.
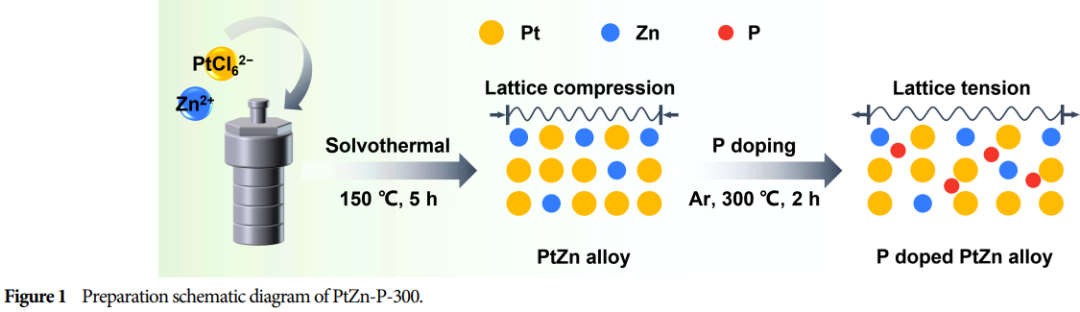
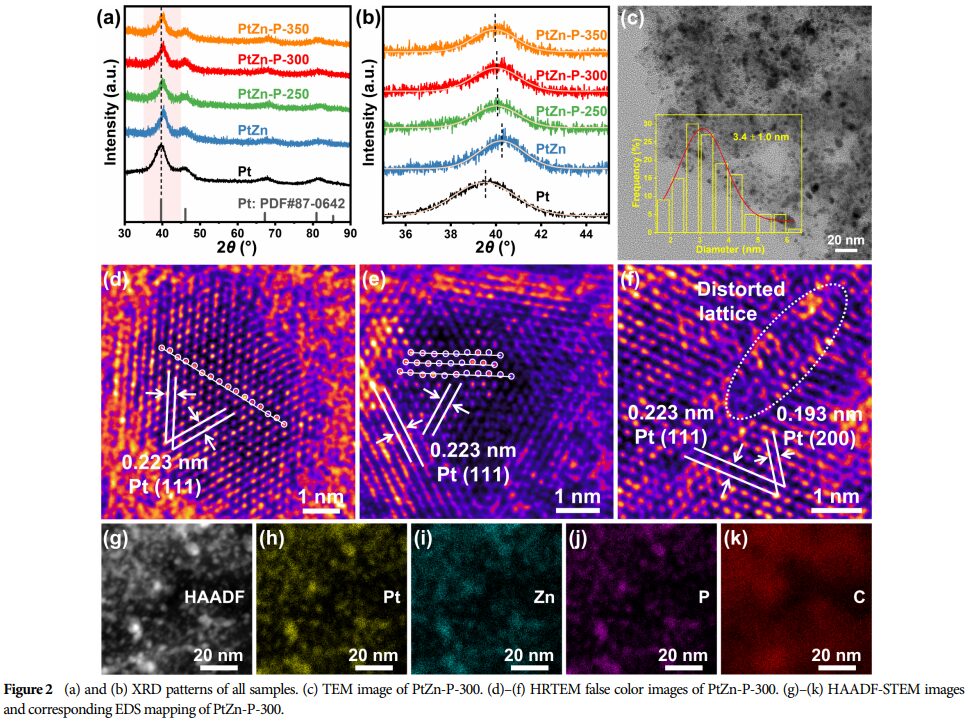
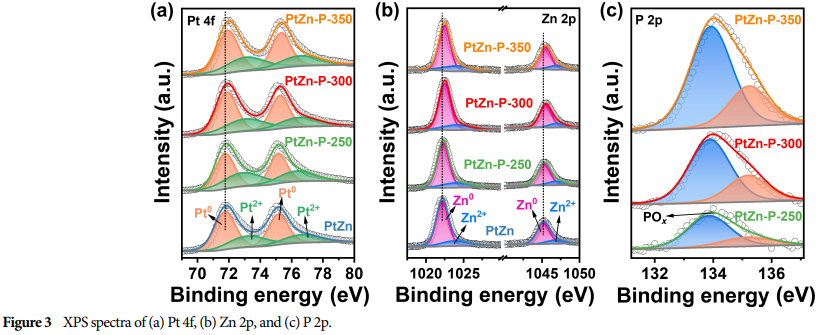
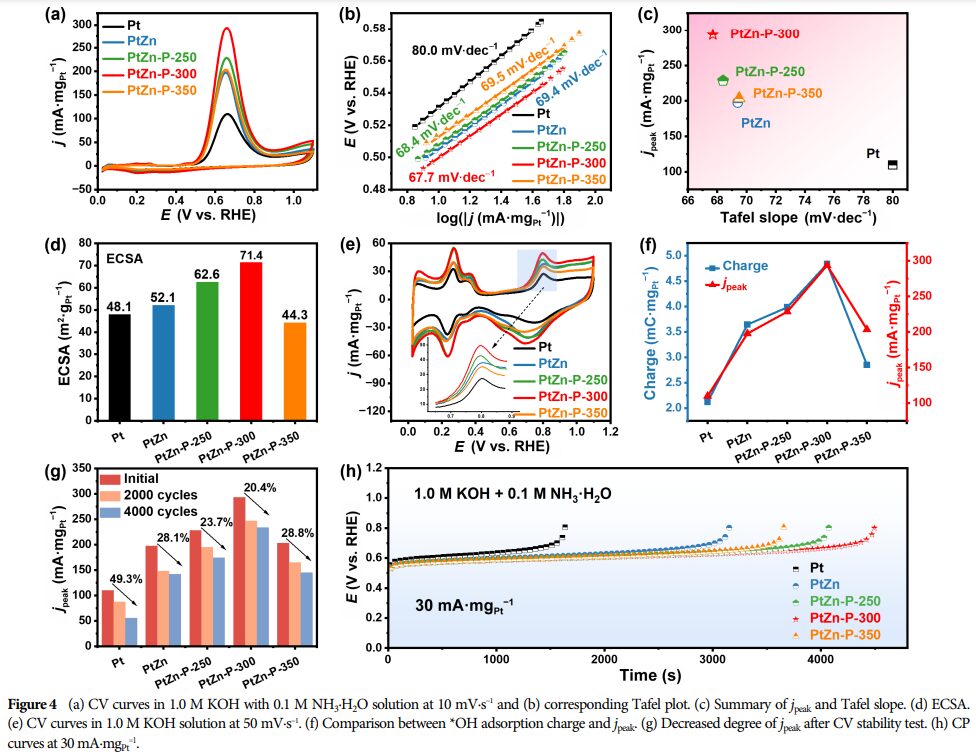

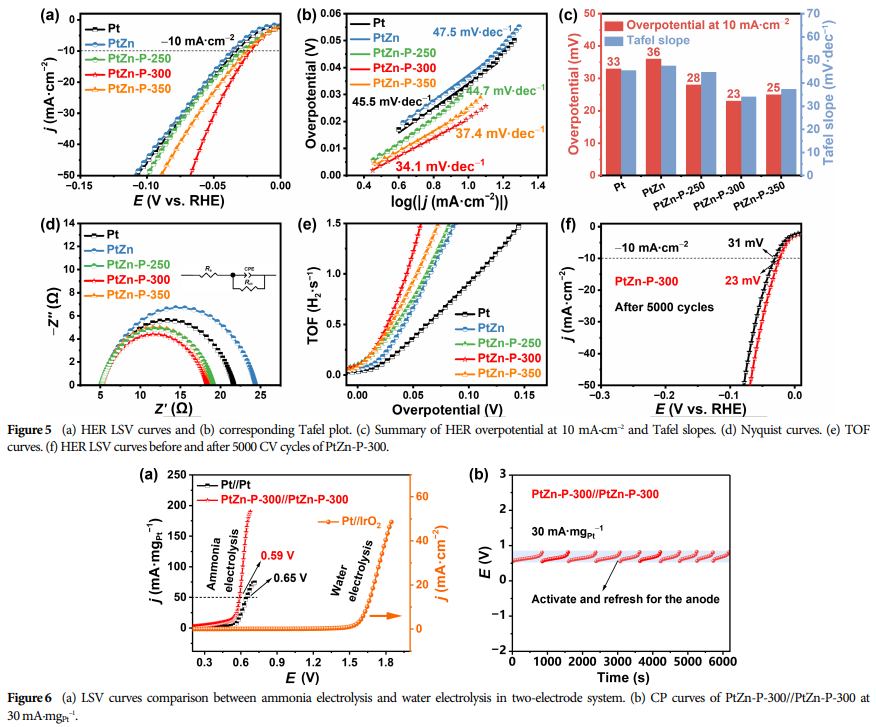
Research Findings: Successfully prepared P-doped PtZn alloy nanoparticles as catalysts, adjusting the lattice strain of platinum and the electronic interactions between phosphorus with platinum/zinc through controlling the doping amount.
This catalyst exhibits excellent dual-function catalytic performance in AOR and HER, indicating potential application prospects.
This article reveals the impact of doping on the lattice strain and electronic interactions of the catalyst through the preparation and characterization of P-doped PtZn alloy catalysts, demonstrating their excellent dual-function catalytic performance in AOR and HER. Additionally, the research provides extensive supplementary data to support its conclusions and potential applications.
This article is provided by the Hydrogen Energy Research Assistant.
https://www.sciopen.com/article/10.1007/s12274-023-5962-x
▲ Disc Electrode Spin Coater








