 As the destructive carbon cycle is blamed for global warming, the feasible electrochemical reduction of carbon dioxide (CO2RR) to form valuable C2+ hydrocarbons and feedstocks has become a hot topic. In this regard, copper-based catalysts have proven to be excellent choices for producing high-energy value-added products through CO2RR. However, the selectivity for C2+ products achieved with copper-based catalysts faces challenges such as high overpotential, slow reaction kinetics, and low selectivity.
As the destructive carbon cycle is blamed for global warming, the feasible electrochemical reduction of carbon dioxide (CO2RR) to form valuable C2+ hydrocarbons and feedstocks has become a hot topic. In this regard, copper-based catalysts have proven to be excellent choices for producing high-energy value-added products through CO2RR. However, the selectivity for C2+ products achieved with copper-based catalysts faces challenges such as high overpotential, slow reaction kinetics, and low selectivity.Furthermore, methods to tune copper interactions are discussed from four key perspectives: single-atom catalysts, interface engineering, metal-organic frameworks, and polymer-infiltrated materials, providing new insights into the selectivity for C2+ products. Finally, major challenges are outlined, and potential prospects for rationally designing robust catalysts for CO2RR are proposed. Combining catalytic design with mechanistic understanding is a step towards advancing CO2RR technology towards industrial application prospects.

01 Reaction Mechanism and Pathways
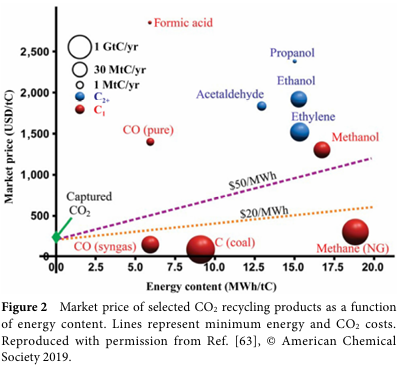
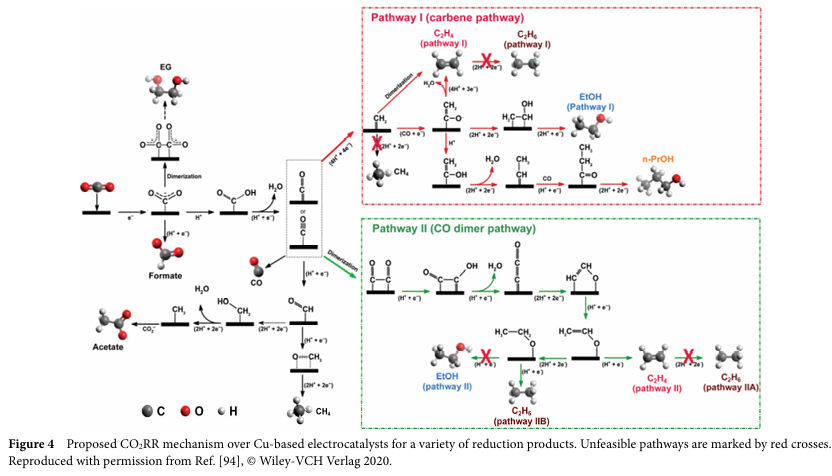
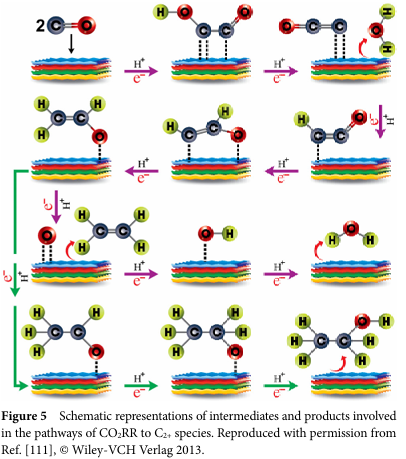
The process of converting CO2RR to C2+ products involves multiple electron transfers and competing reactions, such as the hydrogen evolution reaction (HER). On copper-based catalysts, the formation of C-C bonds is a key step. Researchers have conducted in-depth investigations into possible reaction pathways, particularly regarding the mechanisms of C-C bond formation. These mechanisms include CO dimerization, coupling of CO with CO2, etc., while the specific pathways depend on the structure and surface properties of the electrocatalyst.
02 Strategies for Copper-Based Electrocatalysts
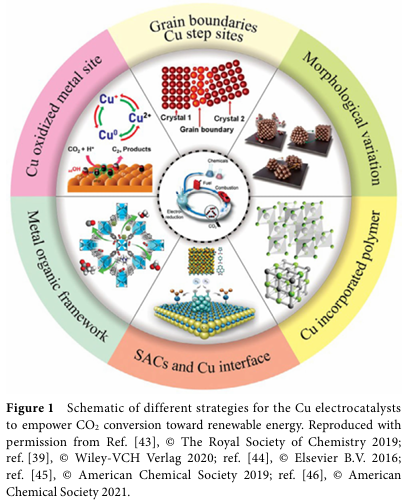
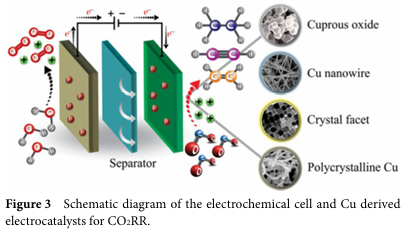
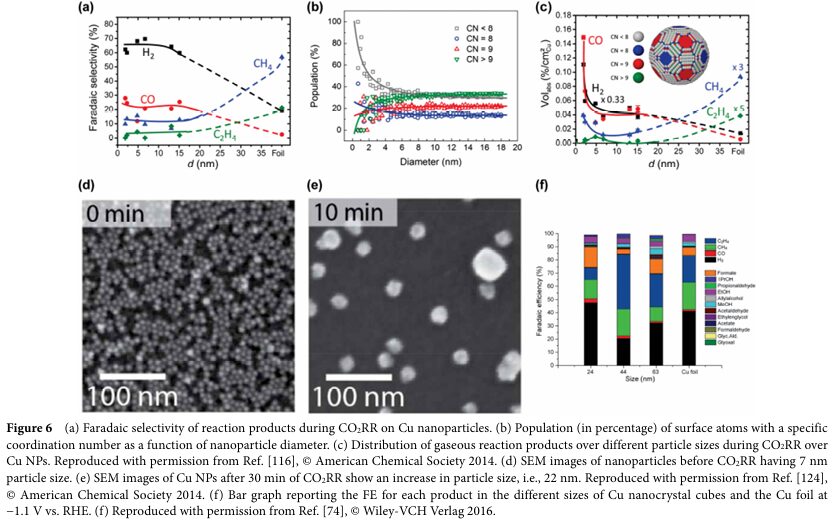
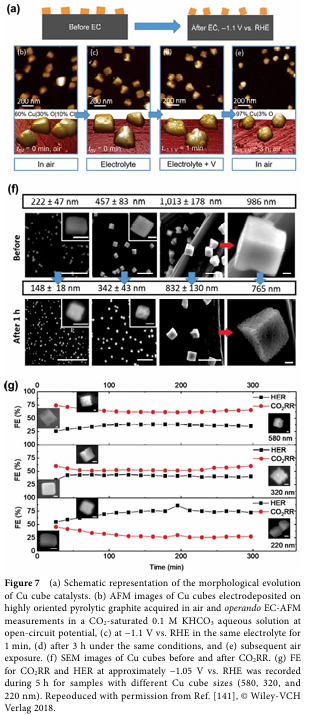
To enhance the selectivity and yield of C2+ products, researchers have adopted various strategies to design copper-based electrocatalysts. Among these, modulating the intermediates of C-C coupling is crucial. This includes adjusting the crystalline phase, morphology, size, and surface modifications of the catalyst. For example, by controlling the oxidation state of the catalyst, the coexistence of Cu+ and Cu0 can be achieved, thereby increasing the yield of C2+ products. Additionally, inducing strain on the surface of Cu nanocrystals through redox strategies can significantly enhance selectivity towards C2+ products.
03 Stability and Activity of Catalysts
Although copper-based electrocatalysts have shown good selectivity for C2+ products in CO2RR, their stability and activity still need further improvement. Researchers have found that issues such as surface reconstruction of the catalyst, carbon deposition, and electrolyte corrosion can affect its performance. Therefore, developing copper-based electrocatalysts with high stability and activity is a current research focus.
04 In Situ Techniques and Mechanistic Elucidation
To gain deeper insights into the mechanisms of CO2RR on copper-based electrocatalysts, researchers have employed various in situ techniques, such as in situ Raman spectroscopy, infrared spectroscopy, and X-ray absorption spectroscopy. These techniques not only allow real-time monitoring of changes on the catalyst surface during the reaction but also reveal the structure and properties of reaction intermediates. By combining theoretical calculations with experimental data, researchers have gained a deeper understanding of the mechanisms of C-C coupling.
05 Illustrated Guide

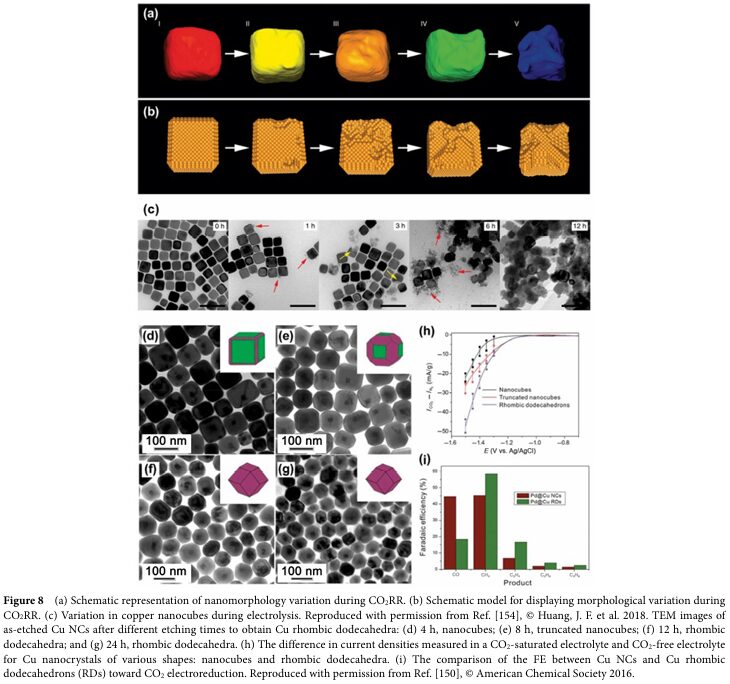
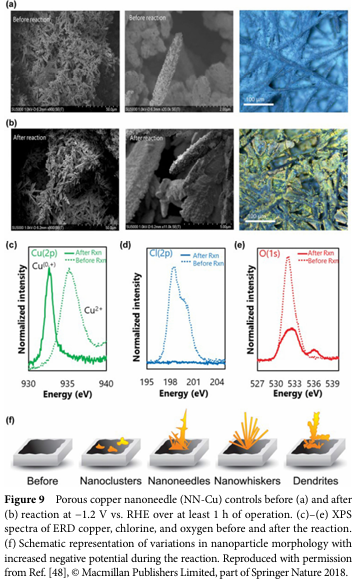
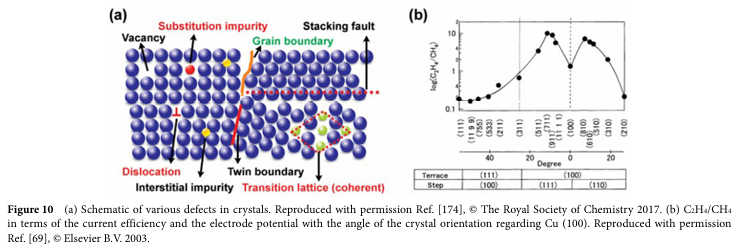
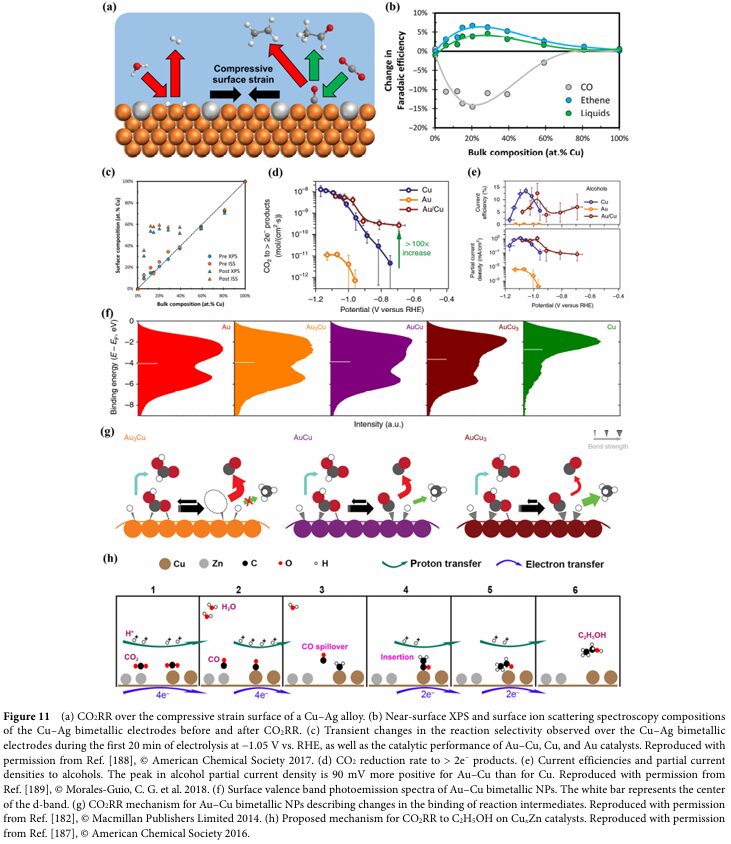
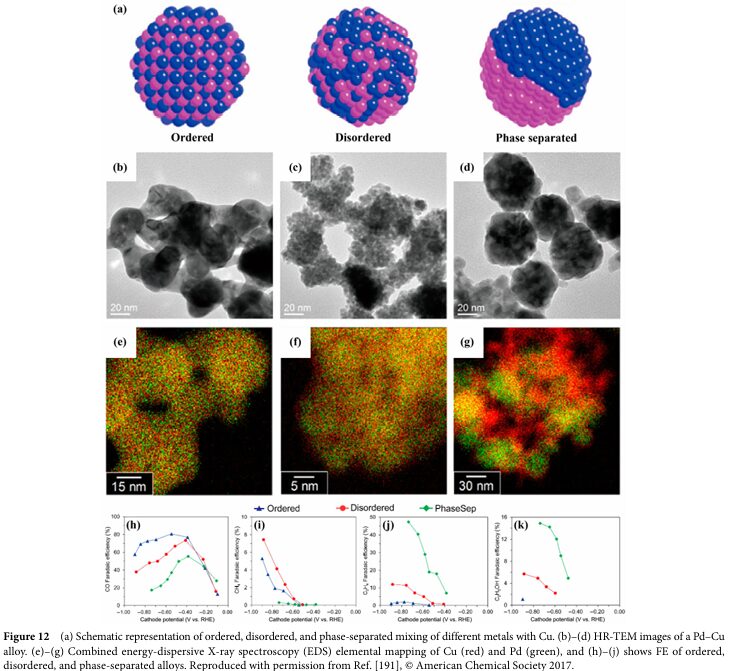
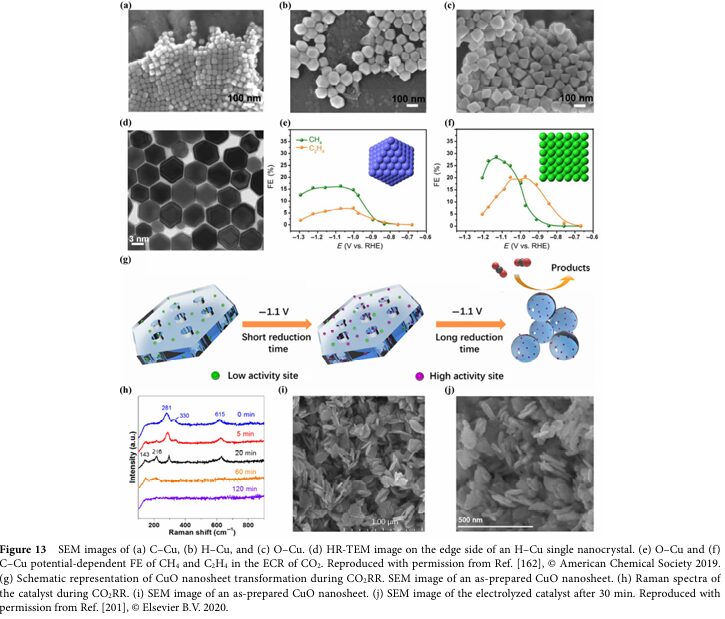
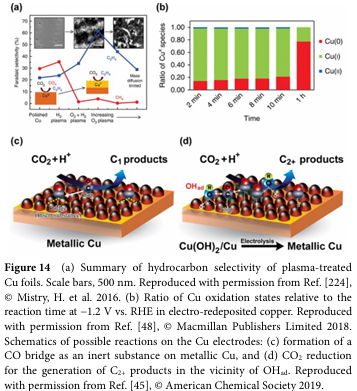
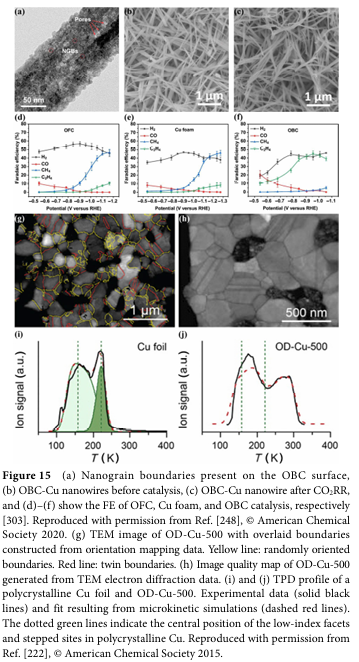
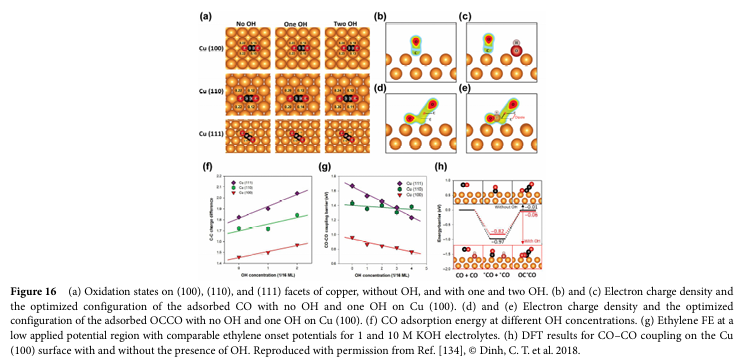
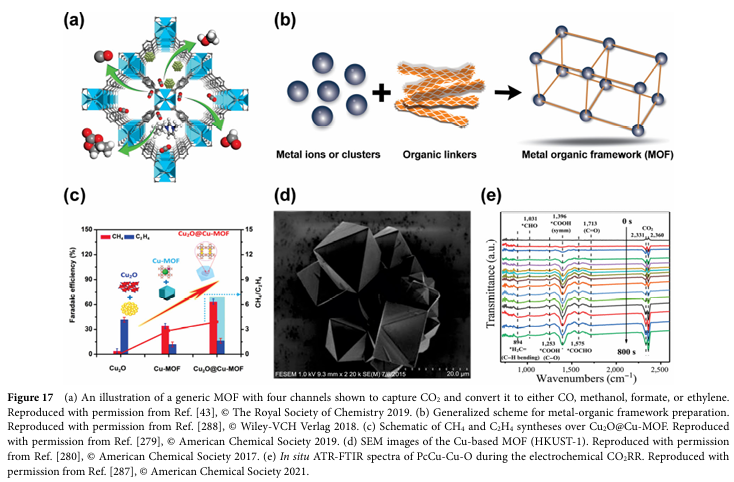
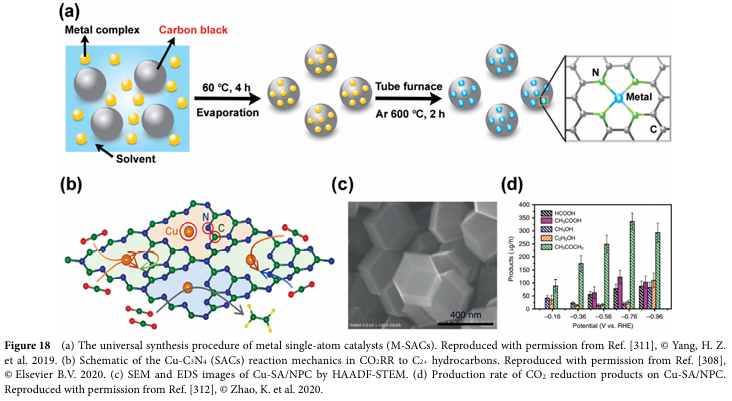
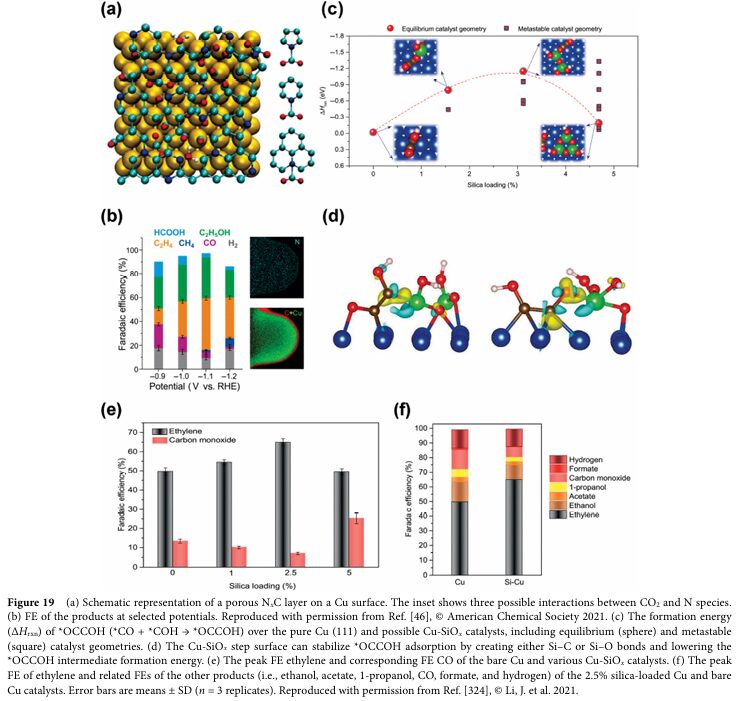

06 Progress and Challenges
In recent years, significant progress has been made in the design and optimization of copper-based electrocatalysts. For example, by precisely controlling the nanostructure and surface properties of the catalyst, high selectivity and yield of C2+ products can be achieved. However, challenges remain, such as the stability, activity of catalysts, and large-scale applications. Additionally, a deeper understanding of the C-C coupling mechanisms will also help guide the design of more efficient catalysts.
07 Future Outlook
-
In the future, researchers will continue to focus on developing copper-based electrocatalysts with higher selectivity and activity to achieve efficient conversion of CO2. At the same time, attention will also be given to the stability and long-term operational performance of the catalysts to meet the needs of practical applications.
-
Moreover, integrating advanced in situ techniques and theoretical calculations will further reveal the mechanisms of CO2RR on copper-based electrocatalysts, providing stronger support for the design and optimization of catalysts.
In summary, the electrochemical reduction of CO2 to C2+ products using copper-based electrocatalysts is one of the hot research topics currently. Through in-depth exploration of reaction mechanisms, optimization of catalyst design, and enhancement of stability and activity, it is expected to achieve efficient conversion and utilization of CO2.
This article is provided by the Hydrogen Energy Research Assistant.