

Written by Wang Cong Edited by Wang Duoyu Formatted by Shui Chengwen
Breast cancer has the highest incidence rate among malignant tumors in women, thus it is referred to as the “pink killer.” According to the global cancer burden data released by the International Agency for Research on Cancer (IARC) in 2020, breast cancer has surpassed lung cancer to become the most prevalent cancer worldwide. In China, the incidence of breast cancer is also increasing year by year, with WHO predicting that there will be as many as 410,000 new cases of breast cancer in China in 2020.More importantly, breast cancer is prone to metastasis. Furthermore, many breast cancer patients are insensitive to chemotherapy, targeted therapy, and immunotherapy, which greatly affects their treatment prognosis.On August 8, 2022, researchers from the Medical University of Vienna published a study titled:Trastuzumab deruxtecan in HER2-positive breast cancer with brain metastases: a single-arm, phase 2 trial in the prestigious medical journal Nature Medicine.This phase 2 clinical trial confirmed that the targeted HER2 antibody-drug conjugate (ADC) drug Trastuzumab deruxtecan can effectively treat and even eliminate brain metastases in HER2-positive breast cancer patients.This study opens up new avenues for oncology research and targeted therapy and is a pioneering study in the treatment of brain metastases from cancer. Trastuzumab deruxtecan, marketed as Enhertu®, abbreviated as T-DXd, is anantibody-drug conjugate (ADC) developed in collaboration between AstraZeneca and Daiichi Sankyo, which received FDA approval in 2019 for the treatment of unresectable or metastatic HER2-positive breast cancer patients. This ADC drug links the HER2 monoclonal antibody Trastuzumab (trastuzumab) with a topoisomerase I inhibitor exatecan derivative (DXd), thereby blocking the HER2 protein while exerting the anticancer effect of the chemical toxin.
Trastuzumab deruxtecan, marketed as Enhertu®, abbreviated as T-DXd, is anantibody-drug conjugate (ADC) developed in collaboration between AstraZeneca and Daiichi Sankyo, which received FDA approval in 2019 for the treatment of unresectable or metastatic HER2-positive breast cancer patients. This ADC drug links the HER2 monoclonal antibody Trastuzumab (trastuzumab) with a topoisomerase I inhibitor exatecan derivative (DXd), thereby blocking the HER2 protein while exerting the anticancer effect of the chemical toxin. The cost of Enhertu is approximately $14,000 per month.This phase 2 clinical trial involved 15 patients (14 females and 1 male) who all had HER2-positive breast cancer with brain metastases. The research team used this ADC drug for treatment.Breast cancer is the most common cancer among women, but men can also develop breast cancer (male breast cancer accounts for less than 1% of breast cancer cases), and 15% of breast cancers are HER2-positive, which are prone to metastasis, with 50% of them metastasizing to the brain.The clinical trial results showed that after treatment, 11 out of 15 patients (73.3%) had a reduction in their brain metastases, with 2 patients (13.3%) experiencing complete disappearance of their brain metastases.
The cost of Enhertu is approximately $14,000 per month.This phase 2 clinical trial involved 15 patients (14 females and 1 male) who all had HER2-positive breast cancer with brain metastases. The research team used this ADC drug for treatment.Breast cancer is the most common cancer among women, but men can also develop breast cancer (male breast cancer accounts for less than 1% of breast cancer cases), and 15% of breast cancers are HER2-positive, which are prone to metastasis, with 50% of them metastasizing to the brain.The clinical trial results showed that after treatment, 11 out of 15 patients (73.3%) had a reduction in their brain metastases, with 2 patients (13.3%) experiencing complete disappearance of their brain metastases.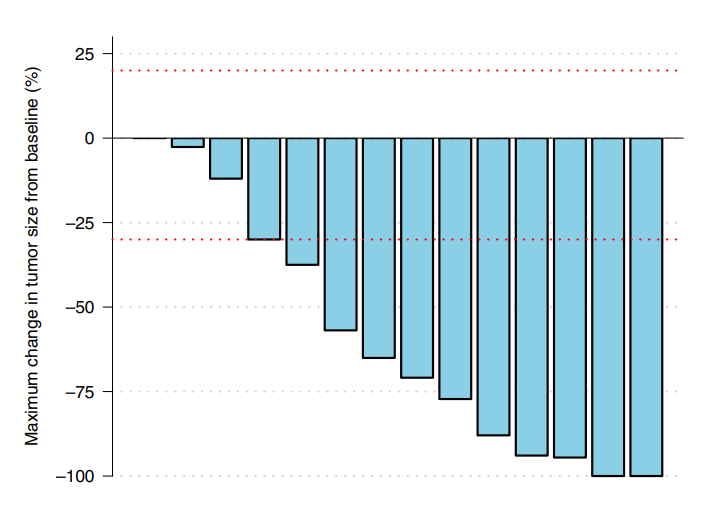 In addition to these extremely positive treatment results, the research team also found that the drug has good tolerability, with no deterioration in brain function or quality of life among participants during treatment.The research team stated that this latest study opens up new avenues for clinical research and treatment of brain metastases from breast cancer, and further studies will be conducted to explore its effects in treating metastases from other types of cancer.It is worth mentioning that on June 5, 2022, the New England Journal of Medicine (NEJM) published a phase 3 clinical trial led by Dr. Shanu Modi from the Memorial Sloan Kettering Cancer Center on Trastuzumab Deruxtecan for treating HER2 low-expressing (HER2-Low) advanced breast cancer patients.This phase 3 clinical trial showed that compared to patients receiving standard chemotherapy,Trastuzumab Deruxtecan extended the progression-free survival of patients with HER2 low-expressing advanced breast cancer by approximately 50% and overall survival by 40%.
In addition to these extremely positive treatment results, the research team also found that the drug has good tolerability, with no deterioration in brain function or quality of life among participants during treatment.The research team stated that this latest study opens up new avenues for clinical research and treatment of brain metastases from breast cancer, and further studies will be conducted to explore its effects in treating metastases from other types of cancer.It is worth mentioning that on June 5, 2022, the New England Journal of Medicine (NEJM) published a phase 3 clinical trial led by Dr. Shanu Modi from the Memorial Sloan Kettering Cancer Center on Trastuzumab Deruxtecan for treating HER2 low-expressing (HER2-Low) advanced breast cancer patients.This phase 3 clinical trial showed that compared to patients receiving standard chemotherapy,Trastuzumab Deruxtecan extended the progression-free survival of patients with HER2 low-expressing advanced breast cancer by approximately 50% and overall survival by 40%. This is the first proof that targeting low-expressing HER2 proteins with drugs can yield cancer treatment effects. About half of the breast cancer patients previously considered HER2-negative are actually HER2 low-expressing (HER2-low) breast cancer, which means they can benefit from this ADC drug. This opens up new treatment possibilities for thousands of advanced breast cancer patients.Paper links: https://www.nature.com/articles/s41591-022-01935-8https://www.nejm.org/doi/full/10.1056/NEJMoa2203690?query=featured_homeCover image source:123rf
This is the first proof that targeting low-expressing HER2 proteins with drugs can yield cancer treatment effects. About half of the breast cancer patients previously considered HER2-negative are actually HER2 low-expressing (HER2-low) breast cancer, which means they can benefit from this ADC drug. This opens up new treatment possibilities for thousands of advanced breast cancer patients.Paper links: https://www.nature.com/articles/s41591-022-01935-8https://www.nejm.org/doi/full/10.1056/NEJMoa2203690?query=featured_homeCover image source:123rf

Copyright Statement/DisclaimerThis article is an authorized reprint, and the copyright belongs to the owner.It is for the cautious reference of interested individuals only, not for commercial, medical, or investment use.Friends are welcome to criticize and correct! Thank you sincerely!The images and videos in the text are authorized original works, or from the WeChat public image library, or taken from the company’s official website/network used under the CC0 protocol, copyright belongs to the owner.For any questions, please contact us (Phone: 13651980212. WeChat: 27674131. Email: [email protected]). Thank you sincerely!
Recommended Reading
- After the Aβ hypothesis was “hammered,” Merck, which left for 4 years, was drawn back again, buying a product whose target is still unknown…
- $5.4 billion, buy! Pfizer, which paused for less than 3 months, has started premium acquisitions again…
- 104 Chinese new drug companies set sail overseas! Good news frequently reported in the ADC track! Kelun Bo Tai’s momentum surpasses the old leader Shiyao | Drug Times Going Abroad Series
- Dialogue with RNA-targeting emerging company SynerK: Looking at the success of Alnylam’s APOLLO-B in the development of RNAi drugs
- Four months in advance! FDA approves ADC drug Enhertu (DS-8201) for the treatment of “HER2 low-expressing” breast cancer patients, market expected to expand fivefold!
- Seven consecutive drug trial failures! New drug development requires perseverance, but also the wisdom of “timely loss”
- A lengthy article: From theory to production, understand mRNA-LNP formulation technology in one article
- Drug Times Live Note Issue 111 | Regulations and guidelines related to the development of pediatric drugs in Europe and America
- 2022 first half report released! Boehringer Ingelheim maintains steady growth, solidifying its R&D pipeline!
- Heavyweight | Nature Medicine published an article, Bangyao Bio’s BRL-101 gene therapy for thalassemia patients has been free from blood transfusion dependence for over 2 years
