

Academic Paper Sharing
The Atomic-Level Structure of Cementitious Calcium Aluminate Silicate Hydrate
DOI:10.1021/jacs.0c02988
Summary / Abstract
Despite the extensive use of aluminum-rich blended cements for over 30 years, the structural nature of aluminum in the primary hydration product, calcium aluminate silicate hydrate (C-A-S-H), remains unclear. Through first-principles calculations, we predict that aluminum will incorporate into bridging sites of linear silicate chains, and at high Ca:Si and H₂O ratios, the stable coordination number of aluminum is 6.
Specifically, we predict that the silicate-bridging [AlO₂(OH)₄]⁵⁻ complex, stabilized by hydroxyl ligands and charge-balancing calcium ions in the interlayer space, is favorable. This structure was then experimentally validated through one-dimensional and two-dimensional dynamic nuclear polarization-enhanced ²⁷Al and ²⁹Si solid-state NMR experiments. Notably, we assign the narrow ²⁷Al NMR signal at 5 ppm to the silicate-bridging [AlO₂(OH)₄]⁵⁻ site, indicating that this signal correlates with the ²⁹Si NMR signal of silicates in C-A-S-H, contradicting the traditional view of attributing it to the “third aluminum hydrate” (TAH) phase. Therefore, we conclude that TAH does not exist. This resolves the long-standing issue regarding the position and nature of six-coordinate aluminum observed in C-A-S-H samples through ²⁷Al NMR.
Introduction / Research Background
Methods and Issues for Reducing the Carbon Footprint of Concrete: The partial replacement of Portland cement with supplementary cementitious materials (SCMs) is an effective way to reduce the carbon footprint of concrete. Many SCMs are aluminum-rich, and their primary hydration product when mixed with cement is calcium aluminate silicate hydrate (C-A-S-H). However, the addition of SCMs can reduce the reactivity of cement, prolong the setting time of concrete, and decrease its workability.
Structural Characteristics of C-S-H and C-A-S-H: In the absence of SCMs, the C-S-H generated from the hydration of ordinary Portland cement has a variable stoichiometry, with an average Ca:Si ratio of about 1.7 and an H₂O:Si ratio of about 1.8. It has a layered structure similar to tobermorite, containing linear silicate chains, a Ca-O main layer, and disordered interlayer regions. C-A-S-H is the product of aluminum incorporation into the C-S-H structure, typically with Al:Si ≤ 0.25, and is a massively produced artificial material, with an estimated production of about 2 billion tons in 2018.
Current Research Status of Aluminum in C-A-S-H: Solid-state ²⁷Al NMR can identify three types of aluminum species in C-A-S-H samples: tetrahedral Al (IV), pentahedral Al (V), and octahedral Al (VI). Among them, Al (IV) substituting for bridging silicate species has been confirmed, while Al (V) is believed to exist in the interlayer, compensating for the charge of tetrahedral aluminum in the silicate chain or binding to the surface of C-A-S-H. When the Ca:Si ratio is 1.0 or higher, Al (VI) associated with C-A-S-H shows a significant narrow signal around 5 ppm in ²⁷Al NMR. As the Ca:Si ratio increases, the proportion of Al (VI) rises, becoming the dominant species when the Ca:Si ratio exceeds 1.5.
Controversy Surrounding Al (VI) Research: For the 5 ppm signal corresponding to Al (VI) species, it was initially thought to substitute for Ca²⁺ in the main CaO layer of C-S-H, but Andersen et al. questioned this through ¹H – ²⁷Al cross-polarization magic angle spinning (CP MAS) NMR experiments, suggesting that this signal corresponds to AlOₓ(OH)₆₋ₓ⁽³⁺ˣ⁾⁻ species. Since this signal disappears at high temperatures (>70°C) while the silicate framework remains unaffected, they attributed it to the “third aluminum hydrate” (TAH) phase, which is believed to consist of chemically disordered aluminum hydroxide or calcium aluminate hydrate, potentially existing independently on the surface of C-A-S-H or penetrating into the interlayer regions. However, apart from solid-state ²⁷Al NMR, no other methods have clearly identified the TAH phase, hindering the understanding of how aluminum species alter cement performance.
Objective of This Study: This study aims to determine the atomic-level structure of C-A-S-H through a combination of theoretical calculations and experimental NMR. The researchers extended the C-S-H brick model to construct the C-A-S-H structural unit, utilizing dynamic nuclear polarization (DNP) enhanced solid-state MAS NMR spectra to obtain experimental evidence and employing density functional theory (DFT) calculations to study the relevant structures and chemical shifts, thereby clarifying the structure and presence of aluminum in C-A-S-H.
Methods / Research Methods
01
Model
Model Extension: The extended C-S-H brick model was used to construct the C-A-S-H structure, allowing for the incorporation of aluminate species. During the construction process, different coordination forms of aluminate were introduced into the C-A-S-H structure by adjusting the structural units.
Aluminum Atom Addition and Coordination Formation:
To obtain the geometries of six-coordinate and five-coordinate structures, one SiO₂ unit was removed from the model, and aluminum atoms (in the form of Al (OH)₂⁺) were added at bridging sites. This addition interacts with interlayer calcium ions to compensate for charge, forming the corresponding coordination structure.
To achieve a tetrahedral structure, AlO (OH) units were added.
Determination of Water Molecule Positions: The initial positions of water molecules were taken from 14Å tobermorite. Additionally, based on previously reported optimized positions of C-S-H structural units, extra water molecules were added to improve the distribution of water in the structure.
Adjustment of Ca:Si Ratio and Charge Compensation:
By removing bridging silicate tetrahedra, the Ca:Si ratio of C-A-S-H can be increased. Further addition of calcium at bridging sites can further increase the Ca:Si ratio to meet the experimental requirement for a high Ca:Si ratio (Ca:Si > 1.5).
To achieve charge compensation, calcium ions and hydroxyl ions were added to the interlayer. These ions play a role in balancing the charge, ensuring the stability of the entire C-A-S-H structure.
02
Simulation
1) DFT Calculations:
Using the Quantum Espresso software package, the ultra-soft pseudopotential with van der Waals corrections (DFT-D2 method) was employed, with a Monkhorst-Pack k-point grid (2×2×1) and a plane-wave cutoff energy set to 80 Ry for structural optimization. To ensure calculation accuracy, a denser grid was sometimes used to check structural convergence.
PBE0 hybrid functional (with an exact exchange fraction of 0.25) was used for calculations, yielding results highly consistent with the PBE-D2 method (with an error within 0.01 eV). Supercell calculations (containing two structural units) verified that it had no effect on the local relaxation of the studied geometric structure.
The structure was visualized using VESTA software. By comparing the calculated isotropic shielding parameters with experimental ²⁷Al chemical shifts of α-alumina (α-Al₂O₃), andalusite, sillimanite, calcium aluminate (Ca₃Al₂O₆), hexahydrate calcium aluminate (Ca₃Al₂(OH)₁₂), and γ-alumina (γ-Al(OH)₃), the ²⁷Al chemical shift calculations were calibrated; ²⁹Si chemical shifts were calibrated using α-quartz (α-SiO₂), andalusite, sillimanite, and belite. The GIPAW method was used to calculate isotropic shielding parameters and the ²⁷Al quadrupole coupling tensor (with the ²⁷Al quadrupole moment set to 0.1403 b). The maximum deviations of the calculated ²⁷Al chemical shifts and quadrupole coupling constants were 4 ppm and 2 MHz, respectively. The isotropic resonance center δiso of ²⁷Al was calculated using a specific formula that considers the isotropic second-order quadrupole shift of the spin I = 5/2 nucleus. Another formula was used to plot the resonance line shape, where R is the root mean square deviation (1 – 2.5 ppm), neglecting the effect of anisotropic second-order quadrupole interactions on line shape asymmetry.
2) Molecular Dynamics Simulation: Classical molecular dynamics simulations were performed using DLPOLY software and an improved CementFF2 force field, with interaction parameters detailed in Supporting Information Section 1.7. The time step was set to 0.2 fs, with a Coulomb cutoff radius of 8.5 Å, using a Nose-Hoover thermostat and barostat, conducted under constant temperature and stress ensembles. The structure was first annealed at 1000 K for 300 ps, followed by equilibrium at 300 K for 1.4 ns.
Supporting Files Available for Learning Reference
03
Experiment
1) Synthesis of C-A-S-H: C-A-S-H samples were prepared using a dropwise synthesis method. Nine-water aluminum nitrate (Al (NO₃)₃・9H₂O) was dissolved in a calcium nitrate solution (100 mL of 0.2 M Ca (NO₃)₂・4H₂O), and under nitrogen protection, sodium silicate solution (Na₂SiO₃) was added dropwise to the stirring solution to prevent carbonate formation. The pH was adjusted to 13.5 using sodium hydroxide (NaOH), and after 3 hours, the precipitate was vacuum filtered and washed with a mixture of ultra-pure water and ethanol (volume ratio 50:50).
2) DNP Enhanced Solid-State NMR:
Most samples were densified by partially drying the C-A-S-H gel on a surface dish, then placed in 3.2 mm zirconia rotors, sealed with PTFE inserts, and capped with zirconia drive caps. AMUPol was typically added (in the form of a 1 M NaOH solution or directly dissolved in the gel).
The experimental field strength was 21.14 T or 9.40 T, equipped with a 3.2 mm MAS DNP probe (¹H/²⁷Al/²⁹Si configuration). Before the experiment, the probe was cooled to below 100 K. The DNP enhancement effect was determined by comparing the intensity ratios of ¹H – ²⁷Al cross-polarization (CP) signals with and without microwave irradiation.
During the ²⁹Si – ²⁷Al refocused dipolar INEPT experiment, ¹H – ²⁹Si CP transfer was first used to hyperpolarize ²⁹Si, followed by re-coupling of ²⁹Si and ²⁷Al nuclei under MAS using the xy – 8 REDOR cycle, which is the same pulse sequence used in the “DNP Enhanced CPTEDOR” experiment by Hanrahan et al. The spectra in Figure 4 were calibrated to the maximum ²⁷Al NMR signal of boehmite set at 7 ppm, while other spectra were calibrated to the maximum Q⁽¹⁾ of the high-resolution ²⁹Si NMR spectrum of C-A-S-H with an initial (Al:Si) ratio of 0.04 set at -79.5 ppm.
Results and Discussion / Research Conclusions
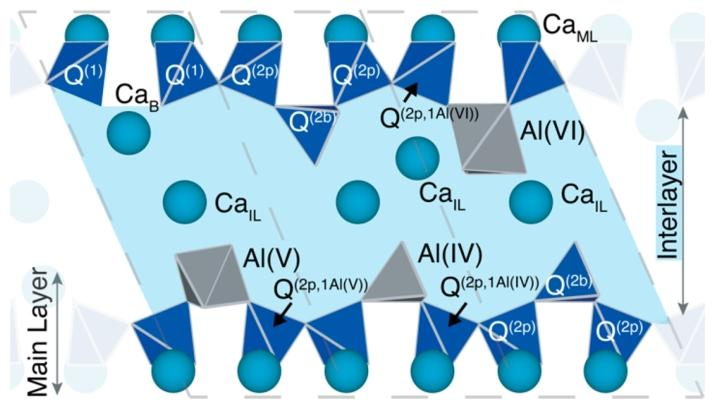
Figure 1. Three types of C-A-S-H structural units representing the layered structure of C-A-S-H. Dashed lines indicate the boundaries of structural units. Calcium atoms are represented as cyan spheres. The interlayers are shown in light blue, indicating the presence of water and hydroxide ions in the interlayer, with calcium ions (CaIL) in the interlayer represented as cyan spheres. Silicate species (dark blue) are labeled according to their connectivity and position. The polyhedral shape of aluminum is shown in gray.
Fundamentals of C-A-S-H Structure:
The C-A-S-H structure is similar to C-S-H, consisting of linear silicate chains and a CaO main layer (CaMI). Various silicate species exist, including “paired” silicates (Q (2p), where the number 2 represents the number of oxygen atoms in the silicate tetrahedron connected to other silicate or aluminate units) and “bridging” silicates (Q (2b)). There are also defect situations, such as Q (1) silicates terminating chains, which commonly occur when bridging calcium sites (CaB) replace Q (2b).
The interlayer contains water, as well as calcium in the form of Ca²⁺, CaOH⁺ ions, or Ca(OH)₂ units, uniformly represented as CaIL species in the figure. This structure can also accommodate tetrahedral, pentahedral, and octahedral aluminate species, which can exist in silicate chains or interlayers. Furthermore, based on the number and type of bonds between silicates and aluminates, Q (n) species are meticulously classified, for example, Q (2p,1Al (VI)) indicates a silicate at a paired position bonded to one other silicate and one Al (VI) octahedron.
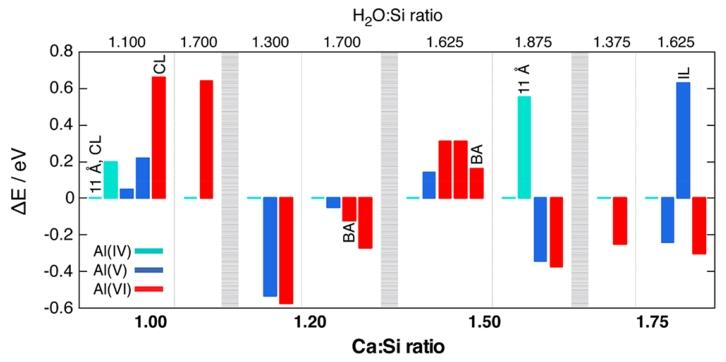
Figure 2. Relative energy of aluminum coordination number (ΔE) in C-A-S-H structural units with different Ca:Si and H₂O:Si ratios. Thick vertical dashed lines divide the results into four regions based on Ca:Si ratios, while thin vertical dashed lines further divide each fixed Ca:Si ratio region into two fixed H₂O:Si ratio regions, forming a total of eight fixed composition panels. Within each panel, the C-A-S-H unit with tetrahedral coordination of aluminum serves as a reference, with a relative energy of ΔE=0. In some cases, the special properties of the optimized structure are labeled: 11 Å, 11 Å tobermorite framework; CL, cross-linked aluminate; BA, aluminum-hydroxyl-silicate bond; IL, interlayer aluminate (as opposed to bridging).
Using DFT calculations, the energy states of aluminate species located at bridging sites of silicate chains or in interlayer spaces were studied in structural units with different Ca:Si ratios. When the Ca:Si ratio is 1.0 (which is lower than the common values in industrial cement formulations), the calculated results align with existing structural models of aluminum in C-A-S-H. At this point, Al (IV) is relatively stable, particularly evident in the 11Å tobermorite framework. In contrast, Al (VI) is energetically higher than its respective reference by over 0.6 eV, indicating that Al (VI) is considered an unstable aluminate species in C-A-S-H with low Ca:Si ratios. During the calculations, two types of cross-linked aluminate structures emerged, where aluminates span across layers connecting parallel silicate chains, a situation that may exist in C-A-S-H with low Ca:Si ratios.
As the Ca:Si ratio increases, it was found that bridging Al (V) and Al (VI) species may be more stable than Al (IV) species. When the Ca:Si ratio reaches its maximum value of 1.75, calculations indicate that Al (VI) is approximately 0.3 eV more stable than the corresponding Al (IV) unit. The stability of Al (V) is intermediate; at low Ca:Si ratios, it is less stable than Al (VI), while at high Ca:Si ratios, it is slightly less stable than Al (VI). Based on these results, the researchers believe that the presence of bridging Al (V) in C-A-S-H cannot be ruled out at any given Ca:Si ratio. Although previous diffraction studies suggested that Al (V) is more likely to exist in the interlayer, the study found that regardless of the initial coordination geometry, interlayer aluminates relax to five-coordinate geometries, and as seen in Figure 2 for Ca:Si = 1.75 and H₂O:Si = 1.625, the relative energy of this structure is relatively high. Therefore, it can be inferred that isolated interlayer aluminates are less stable than bridging aluminates in C-A-S-H.
Influence of Other Structural Features on Stability:
When the Ca:Si ratio exceeds 1.0, no cross-linked aluminates were found in all studied structures. Under such Ca:Si conditions, some optimized Al (VI) structures (marked as “BA” in Figure 2) exhibit hydroxyl silicate bonds, which demonstrate Brønsted acidity and show minimal energy loss, less than 0.1 eV (local structure shown in Figure S6). This bonding mode has been previously reported in tetrahedral coordinated aluminates on silicate surfaces.
As the H₂O:Si and Ca:Si ratios increase, the number of hydroxyl groups in the structure also increases correspondingly. The optimized structures show a trend where hydroxyl groups tend to enter the first coordination shell of bridging Al (VI) aluminate species. Thus, the researchers infer that hydroxyl ligands contribute to stabilizing Al (VI) units in silicate chains. For example, at a Ca:Si ratio of 1.0, the lower hydroxyl content (OH⁻:Si ratio of 0.2, while at Ca:Si ratio of 1.75, this ratio is 1.25) makes bridging Al (IV) more favorable than Al (VI), which aligns with previous experimental and theoretical studies.
Regarding the influence of water on the stability of Al (VI), the calculated results indicate that, except for the case of Ca:Si ratio of 1.5, the relative stability of Al (VI) is generally independent of the local water structure. At a Ca:Si ratio of 1.5, Al (VI) has water ligands [AlO₂(OH)₃(H₂O)]⁴⁻. In this case, the higher water content stabilizes the water molecules coordinated with Al (VI) through a more extensive hydrogen bonding network (see Figure S5). In contrast, stable tetrahedral coordinated aluminates do not accept any water molecules even at H₂O:Si ratios as high as 1.7. When the Ca:Si ratio is 1.75, stable Al (VI) coordinates with four hydroxide ions and two oxygen atoms from silicate tetrahedra, and removing water molecules from the interlayer does not lead to its destabilization. These results differ from previous reports suggesting that Al (VI) can exist in C-A-S-H as long as it has a strong association with water molecules.
Influence of Al:Si Ratio:
Generally, the amount of aluminum absorbed by Portland cement is limited, with an Al:Si ratio of approximately 0.1, which can approach 0.3 when adding aluminum oxide-rich components or used for alkali-activated materials. The Al:Si ratios of the structures involved in this study are 0.20 or 0.25.
By arranging C-A-S-H structural units adjacent to C-S-H structural units, structures with lower Al:Si ratios can be constructed, as detailed in Supporting Information Section 1.4. For a structure with Ca:Si = 1.55 and Al:Si = 0.11, it was found that ΔE for Al (VI) is -0.8 eV. This indicates that the lower overall aluminum concentration reduces the competition for OH⁻ needed to stabilize Al (VI), thereby allowing Al (VI) to achieve a greater degree of stability, with each aluminate unit being more stable than the structures described in Figure 2.
Model Validation: The nano-mechanical properties of some C-S-H and C-A-S-H structural units were calculated and discussed in Supporting Information Section 1.5. The results show that the model accurately reflects the nano-mechanical properties determined by Geng et al. through synchrotron-based high-pressure X-ray diffraction experiments, further validating the model’s effectiveness.
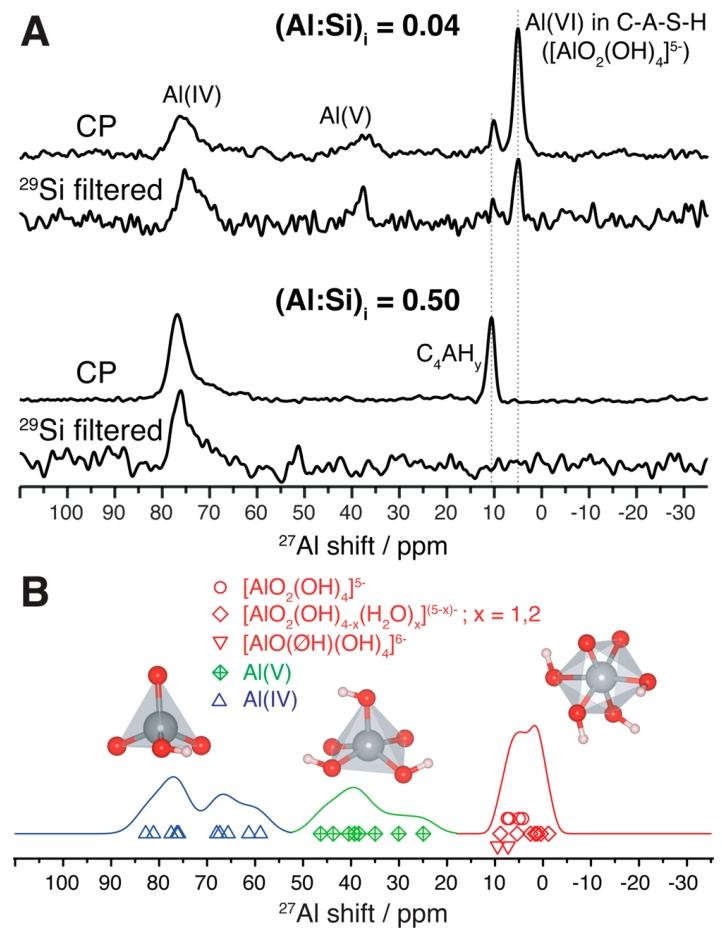
Figure 3. (A) DNP-enhanced ²⁷Al CP MAS spectrum, 21.14 T, 100 K, 12.5 kHz MAS, for synthesized C-A-S-H samples with Ca:Si ratio of 2.0 and initial Al:Si ratios of 0.04 (top) and 0.50 (bottom), with and without the insertion of a ²⁹Si filter. CP selects the nuclear signal in the proton-rich phase. Additionally, the ²⁹Si filter selects the NMR signals of ²⁷Al nuclei close to the ²⁹Si nuclei. The ²⁹Si filtering is achieved through the refocused dipolar INEPT sequence. C4AHy is the abbreviation for the hydrated AFm phase. (B) The calculated ²⁷Al chemical shifts of the C-A-S-H structure described in Figure 2. The line shapes are plotted based on the calculated isotropic chemical shifts of tetrahedral (green), pentahedral (blue), and octahedral (red) coordinated aluminum. ØH is the abbreviation for aluminum-hydroxyl-silicate bonds.
Challenges and Solutions in Sample Preparation:
The challenge in studying C-A-S-H lies in the complexity of hydrated cement materials, which contain various other phases in addition to C-A-S-H, increasing the uncertainty of the research. To reduce these interferences, this study employed a rapid precipitation method. This method effectively removes unwanted solids such as Ca(OH)₂ and Ca₃SiO₅, which are difficult to avoid with traditional methods, resulting in a relatively pure C-S-H product with a controllable Ca:Si ratio. On this basis, the method was further extended to synthesize C-A-S-H with a controllable Al:Si ratio by adding specific concentrations of aluminum nitrate aqueous solution to the calcium nitrate reagent solution. At the same time, the introduction of sulfate and iron species was completely avoided during the synthesis process, ensuring that the final C-A-S-H product is free from paramagnetic Fe³⁺ impurities, thereby enhancing the resolution and sensitivity of the samples, which are typically affected by these impurities in actual cement preparation samples.
Achieving DNP Enhanced NMR Signals:
To conduct low-sensitivity one-dimensional and two-dimensional ²⁹Si – ²⁷Al solid-state NMR experiments to explore the relationship between Al and Si, it is necessary to enhance the NMR signals. Since the samples were not isotopically enriched with ²⁹Si, the study employed magic angle spinning dynamic nuclear polarization (MAS DNP) technology to achieve signal enhancement. Specifically, a small amount of the organic biradical AMUPol was added to the sample, which is specially designed to efficiently transfer a large amount of electron polarization to protons in the sample. During the magic angle spinning experiment, the sample was cooled to 100K, at which point proton spin diffusion spontaneously transfers the enhanced polarization throughout the sample. Once the sample is hyperpolarized, the enhanced ¹H magnetization can be transferred to ²⁹Si or ²⁷Al nuclei through cross-polarization (CP).
In aqueous formulations, d₈-glycerol is typically added to assist in forming a glassy matrix. However, the study found that glycerol can convert the Al (VI) species associated with the 5 ppm ²⁷Al NMR signal into Al (IV) and Al (V) species (related content can be found in Supporting Information Figure S11), a phenomenon attributed to the dehydrating effect caused by the hygroscopic nature of glycerol. Fortunately, by directly dissolving AMUPol in the sample, the low-temperature protective characteristics of C-A-S-H itself can achieve sufficient signal enhancement. Under a 9.40T magnetic field, the DNP enhancement factor for ²⁷Al and ²⁹Si is typically around 40; under a 21.14T magnetic field, this factor is about 3. To exceed a DNP enhancement factor of 4 under a 21.14T magnetic field, it is necessary to add a basic (pH around 13) NaCl solution, possibly due to its dielectric effect. Unlike glycerol, after adding the NaCl solution, most of the 5 ppm Al (VI) signal is retained, and this formulation can increase the DNP enhancement factor to about 8.
DNP Enhanced ¹D ²⁷Al CP MAS NMR Experimental Results:
Figure 3A shows the results of two different DNP enhanced ¹D ²⁷Al CP MAS NMR experiments conducted on C-A-S-H samples with a Ca:Si ratio of 2.0 and different initial (Al:Si) ratios. Here, “initial” refers to the Al:Si ratio in the reagent solution, as the study did not determine the Al:Si ratio in the C-A-S-H product. When (Al:Si) i ratio is 0.04, the conventional CP spectrum shows four different aluminum species, which cluster around approximately 75 ppm, 40 ppm, and 5 ppm, corresponding to the well-known Al (IV), Al (V), and Al (VI) aluminum species’ chemical shifts. In Figure 3B, the isotropic ²⁷Al chemical shifts calculated from the structures of different types of Al using the GIPAW method are presented (including contributions from the isotropic second-order quadrupole shift derived from the calculated quadrupole coupling tensor). The signals in the Al (IV) and Al (V) regions are broader, consistent with the expected quadrupole coupling constants (usually exceeding 3 MHz), which lead to anisotropic second-order quadrupole broadening, even under magic angle spinning conditions. This broadening is inversely proportional to the magnetic field strength, and under the high magnetic field of 21.14T used in this study, the Al (IV) and Al (V) signal regions do not overlap. When (Al:Si) i ratio is 0.50, Al (V) essentially disappears, and the sharp Al (IV) signal observed at around 72 ppm in some lower Ca:Si ratio C-A-S-H formulations is also absent here.
The quadrupole coupling constant ICq for the Al (VI) signal is relatively low, close to 1 MHz, which alleviates the second-order quadrupole effect, resulting in a narrower peak shape. For the sample with (Al:Si) i = 0.04 under a 21.14T magnetic field, two distinct Al (VI) signals can be discerned, with maxima at 5.0 ppm and 10.1 ppm, respectively. The 5.0 ppm signal is associated with the presence of C-A-S-H, dominating the spectrum of the sample synthesized at (Al:Si) i = 0.04, but disappears in the sample with (Al:Si) i = 0.50. Conversely, in the sample with (Al:Si) i = 0.50, the 5.0 ppm signal is absent, and only a single narrow Al (VI) signal with a maximum at 10.5 ppm is observed. In aluminum-containing cements, narrow signals in the range of 8 – 14 ppm are typically the result of various crystalline Al (VI) phases produced during cement hydration, such as (but not limited to) ettringite, AFm phases, and siliceous hydrogarnet. Since the formulation of this study does not contain sulfates and iron, the 10.5 ppm Al (VI) signal is attributed to the hydroxylated AFm phase Ca₄[Al(OH)₆]₂(OH)₂・yH₂O (y = 4 – 12), whose chemical shift and the low ICq value implied by the peak’s narrowness (approximately 1 MHz) align most closely with experimental observations. The 10.1 ppm signal at (Al:Si) i = 0.04 may also originate from such phases, but the certainty of this attribution is lower. Supporting Information Figure S11 shows the ²⁷Al NMR spectrum of the C-A-S-H gel at (Al:Si) i = 0.04, where efforts were made to minimize its contact with dry air during the preparation of the NMR sample, at which point the 10.5 ppm signal completely disappears. However, even mild drying conditions can lead to the formation of hydroxylated AFm phases and an increase in the proportion of Al (IV) species, while sacrificing the Al (VI) signal at 5 ppm. This indicates that, at least in the case of low Al:Si ratios, the formation of foreign solids such as hydroxylated AFm phases is a decomposition process driven by the loss of structural water.
DNP Enhanced ²⁹Si Filtered ²⁷Al NMR Experimental Results:
The DNP enhanced ²⁹Si filtered ²⁷Al NMR experimental results further confirm the above inferences. In this experiment, the DNP enhanced ¹H polarization was first transferred to ²⁹Si nuclei through CP, and then transferred to ²⁷Al nuclei for detection. The transfer to ²⁷Al was achieved using the refocused dipolar INEPT sequence, mediated by spatial dipolar coupling between ²⁹Si and ²⁷Al nuclei, with the interaction strength inversely proportional to the cube of the distance between the two nuclei (r⁻³). Thus, the dipolar-mediated transfer allows researchers to selectively observe those ²⁷Al nuclei that are close enough to the ²⁹Si nuclei to receive magnetization transfer, filtering out those aluminum species that are too far from the ²⁹Si nuclei to receive magnetization transfer (if ²⁷Al exists in non-silicate phases, this situation would occur).
For the spectra in Figure 3A, a short recoupling interval was chosen (with a detection distance of up to 2.46 Å), allowing magnetization to transfer only between aluminum species directly bonded to silicates from ²⁹Si to ²⁷Al. It can be observed that every signal associated with C-A-S-H appearing in the ordinary ²⁷Al CP spectrum is also present in the ²⁹Si filtered spectrum. Crucially, the 5.0 ppm Al (VI) signal remains after ²⁹Si filtering, indicating that this Al (VI) species exists in a silicate-containing phase, namely C-A-S-H. This finding contradicts the traditional view of attributing this signal to a non-silicate TAH phase.
In contrast, by comparing the CP spectrum and the ²⁹Si filtered spectrum of the sample with (Al:Si) i ratio of 0.50, it can be found that the signals of the hydroxylated AFm phase are eliminated by the refocused dipolar INEPT sequence. This result is entirely consistent with the fact that the hydroxylated AFm phase does not contain silicates. Additionally, the ²⁷Al NMR signal of siliceous hydrogarnet is attributed to Al (VI) around 11 ppm, which contains AlO₆ – SiO₄ bonding patterns. The nearest neighbor silicate filtering eliminates the 10.5 ppm signal, indicating that no siliceous hydrogarnet phase was formed in the aluminum-rich C-A-S-H synthesized by the rapid precipitation method. The chelation of the hydroxylated AFm phase with calcium and the higher aluminum content may lead to the formation of C-A-S-H with a low Ca:(Si + Al) ratio. Compared to the amount of aluminum incorporated, the lower calcium concentration reduces the availability of hydroxyls needed to stabilize Al (VI), thereby favoring the formation of Al (IV), which aligns with experimental observations.
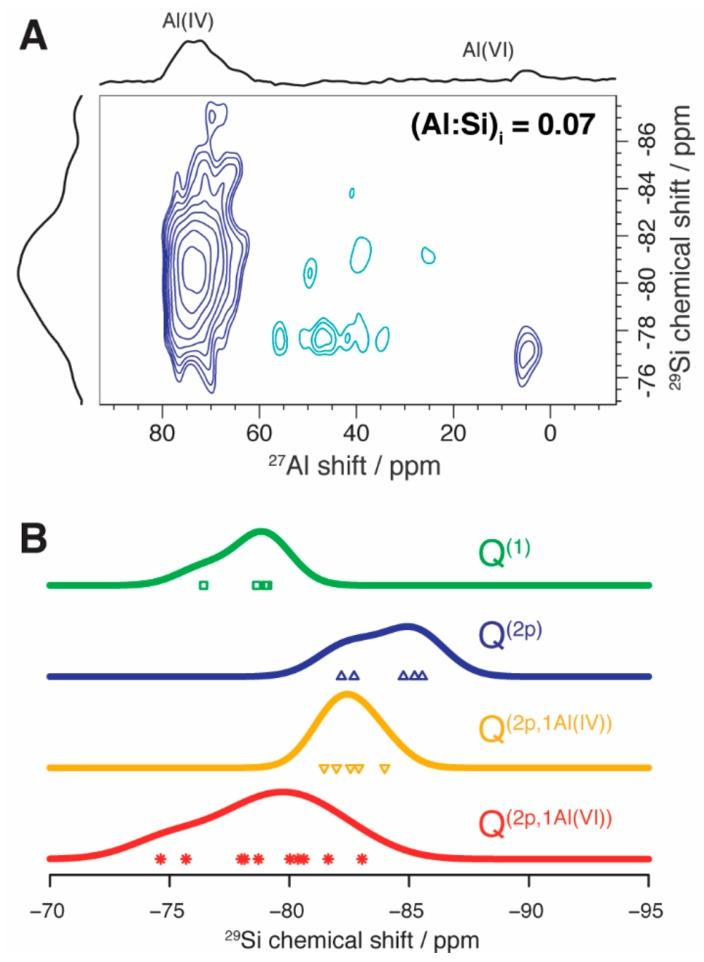
Figure 4. (A) DNP-enhanced 2D {29Si}27Al refocused dipolar INEPT MAS spectrum, 9.40 T, 100 K, 10 kHz MAS, for synthesized C-A-S-H samples with Ca:Si ratio of 2.0 and Al:Si ratio of 0.07. (B) DFT calculated 29Si isotropic chemical shifts of the C-A-S-H structural units described in Figure 2, categorized according to Q(n) species.
Selection of Experimental Objectives and Methods:
To more accurately determine the position of Al (VI) in C-A-S-H, the researchers conducted two-dimensional DNP enhanced ²⁹Si – ²⁷Al refocused dipolar INEPT NMR experiments. This experiment can correlate the ²⁷Al resonance frequency with the ²⁹Si chemical shift, but due to the low concentration of Al and the natural abundance of ²⁹Si being only 4.67%, the experimental sensitivity is extremely low. Therefore, the experiment was chosen to be conducted on C-A-S-H samples with (Al:Si) i ratio of 0.07 at a lower magnetic field of 9.40 T. At this magnetic field, the cross-effect DNP mechanism performs better, and although the second-order quadrupole effect slightly reduces the dimensional resolution of ²⁷Al, it significantly improves sensitivity. Meanwhile, a longer recoupling time (with a detection distance of up to 4.33 Å) further enhances sensitivity.
Analysis of Two-Dimensional Experimental Results:
The two-dimensional DNP enhanced ²⁹Si – ²⁷Al refocused dipolar INEPT correlation spectrum obtained from the experiment (Figure 4A) presents two main correlation peaks. The strongest peak correlates the ²⁷Al in the Al (IV) species with the ²⁹Si chemical shift distributed around -81 ppm, corresponding to the typical tetrahedral Al (IV)-O-Si bonding mode in C-A-S-H. Further investigation reveals that the silicate of this bonding mode tends to be in a paired position and is also bonded to paired silicates in the main chain, marked as Q (2p,1Al (IV)) species.
Analysis of the 5 ppm Signal Correlation Peak:
The other weaker but focused peak is associated with the 5 ppm Al (VI) signal in C-A-S-H. This signal is primarily correlated with silicates having a chemical shift of approximately -77 ppm, significantly lower than that of Q (2p,1Al (IV)) species. Traditionally, this chemical shift falls within the range of Q (1) silicate species, which are commonly found at chain ends. This may suggest a non-bonding association of Al (VI) with the chain ends of silicates, but contradicts DFT results, as DFT indicates that Al (VI) is unlikely to stably exist in the interlayer. Another possibility is that Al (VI) has a bonding association with silicates. It is known that as AlO₄ tetrahedra gradually replace SiO₄ in covalent bond networks, silicate units exhibit a deshielding trend. Studies indicate that the magnitude of this effect also depends on the coordination nature of the aluminate species. From an electrostatic perspective, the Al-O bond length in Al (VI) octahedra is greater than that in Al (IV) tetrahedra, which reduces the covalency of the Al (VI)-O bond and increases the covalency of the bridging Si-O bond, making it relatively shorter than the Al (IV)-O bond. Under otherwise identical conditions, the contraction of Si-O bonds is related to the isotropic deshielding of ²⁹Si nuclei.
Verification of DFT Calculations:
To confirm the above inferences, the researchers calculated the ²⁹Si chemical shifts of Q (2p,1Al) species in the DFT optimized structures, and the results (Figure 4B) confirmed the expected deshielding effect. The average ²⁹Si chemical shift values for Q (2p,1Al (VI)) and Q (2p,1Al (IV)) distributions were calculated to be -79.1 ppm and -82.3 ppm, respectively, with a difference of +3.2 ppm consistent with the maximum values of the ²⁹Si chemical shifts associated with Al (VI) and Al (IV) correlation peaks. The maximum deviation was analyzed to be less than 3 ppm, confirming the significance of this difference. Therefore, the ²⁷Al NMR signal previously attributed to the “third aluminum hydrate phase” is highly likely to correspond to the bridging Al (VI) species in C-A-S-H as a network-forming entity. This atomic-level correlation and the strong correlation of Al (VI) with specific silicate sites make hypotheses such as intercalation or physical adsorption of Al (VI) difficult to establish.
Comparison with Previous Studies and Conclusions:
The study indicates that the bridging Al (VI) hypothesis aligns with previous observations by Andersen et al., without the need to introduce a third (calcium) aluminum hydrate phase. The new ²⁹Si – ²⁷Al correlation NMR data supports the bridging Al (VI) hypothesis, while the TAH hypothesis contradicts it, leading the researchers to conclude that the TAH phase does not exist.
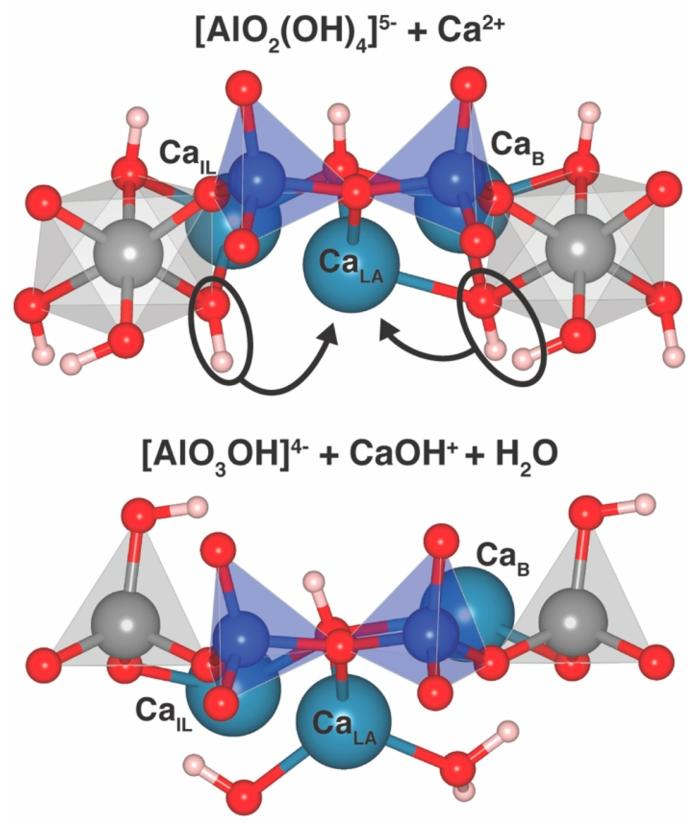
Figure 5. The calcium coordination environment between bridging aluminum species Al (VI) (top) and Al (IV) (bottom) in the DFT optimized structure at Ca:Si = 1.75, H₂O:Si = 1.625. The calcium ion marked as CaLA exhibits stronger Lewis acidity when flanked by Al (IV). The [AlO₂(OH)₄]³⁻ complex can be viewed as a donor of the OH⁻ and H₂O ligands circled in the figure, generating [AlO₃OH]⁴⁻ when these ligands are provided to CaLA. The cutoff value for Ca-O bond lengths is set at 2.5 Å, resulting in an effective coordination number of 3 for CaLA in the upper figure and 4 in the lower figure.
Proposed Equilibrium Reaction for Interconversion:
The characteristics of Al (VI) species in C-A-S-H are closely related to the hydration state of the samples. The study proposes an equilibrium reaction describing the interconversion between bridging Al (IV) and Al (VI) in C-A-S-H: [AlO₂(OH)₄]⁵⁻ + Ca²⁺ ⇌ [AlO₃OH]⁴⁻ + CaOH⁺ + H₂O. Under dehydration conditions, such as low humidity, heating, or the addition of hygroscopic additives, water will leave the C-A-S-H structure, driving this equilibrium reaction to the right. Thermogravimetric analysis (TGA) revealed that C-A-S-H samples with (Al:Si) i = 0.07 begin to lose significant water above 50°C, which aligns with the observation by Andersen et al. that the TAH signal disappears above 70°C, also explaining why the addition of hygroscopic glycerol causes the disappearance of the 5 ppm Al (VI) signal in the ²⁷Al NMR spectrum.
Verification of DFT Optimized Structures:
The DFT optimized structures provide strong support for the above chemical reaction. In the two structures with Ca:Si ratio of 1.75 and H₂O:Si ratio of 1.625, the environments of interlayer calcium near bridging aluminates differ. In the structure containing Al (VI), a specific calcium ion (CaLA) appears to bridge two [AlO₂(OH)₄]⁵⁻ complexes, aligning with the left side of the reaction equation. In the Al (IV) structure, the [AlO₃OH]⁴⁻ tetrahedron and CaLA do not exhibit this bridging configuration, and CaLA, acting as a Lewis acid, will generate new interactions with OH and H₂O, consistent with the right side of the reaction equation. In these structures, the ratio of [AlO₂(OH)₄]⁵⁻ to CaLA is 1:1, meaning that each [AlO₂(OH)₄]⁵⁻ provides one OH and one H₂O ligand when converting to [AlO₃OH]⁴⁻. This links the proposed reaction with DFT results, highlighting the key roles of calcium and water in regulating the aluminum silicate chain structure in C-A-S-H.
Stability Analysis of Two Structures:
Although DFT calculations indicate that the total energy of the Al (VI) environment is lower (ΔE = -0.3 eV), both the Al (IV) and Al (VI) related structures are stable, each residing at local energy minima on the reaction coordinate. Under dehydrated conditions, the free energy of the aforementioned equilibrium reaction is expected to be negative, further indicating the possibility of interconversion and the variation in stability of these two species under different conditions.
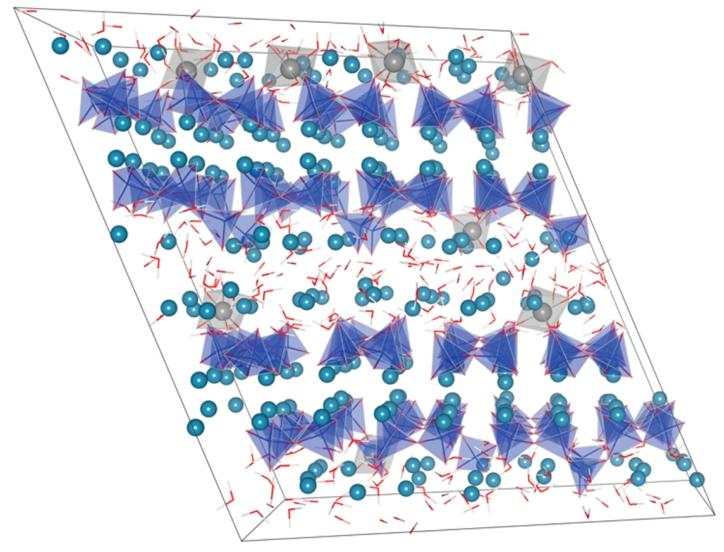
Figure 6. Representative structure of C-A-S-H with a stoichiometry of (CaO)₁.₇(Al₂O₃)₀.₀₅SiO₂(H₂O)₁.₉
Structure Construction and Simulation:
The researchers constructed a representative bulk structure of C-A-S-H that includes Al (IV), Al (V), and Al (VI) at bridging positions based on NMR results. This structure is built on defective 14Å tobermorite “bricks,” with dimensions of 4×4×2 units in the a, b, and z directions, respectively. By juxtaposing different C-S-H or C-A-S-H structural units, this bulk structure was formed. Classical molecular dynamics simulations were performed to equilibrate the constructed structure. The simulation results show that the bridging Al (VI) in the bulk structure remains stable within 2 ns at 300K and 1 atm, indicating that the structure retains the fundamental features inferred from the analysis of individual structural units.
Structural Composition and Characteristics:
The Ca:Si, Al:Si, and H₂O:Si ratios of this structure are 1.7, 0.05, and 1.9, respectively. The average length of aluminum silicate chains is 3.17, consisting of dimers (disilicate units), pentamers, and octamers. This is close to the findings of Dai et al. on C-A-S-H with Al:Si = 0.05 after 28 days of hydration of white Portland cement (average chain length of 3.21, but this study only considered bridging Al (IV)). In C-S-H, Thomas et al. observed that 23% of calcium charge is balanced by hydroxyl groups, while in this structure, this proportion is approximately 22%. After equilibration, the percentages of Al (IV), Al (V), and Al (VI) in the structure are 0%, 25%, and 75%, respectively, corresponding to an average coordination number of 5.75. In the initial structure, all aluminum exists in tetrahedral coordination. During the structural relaxation process using a non-reactive force field, the increase in aluminum coordination number is directly related to the local availability of nearby hydroxyl species. If OH⁻ is present nearby, the bridging [AlO₃OH]⁴⁻ configuration will maintain tetrahedral coordination or convert to bridging Al (V) or Al (VI) geometries; while if the initial configuration is [AlO₂(OH)₂]³⁻ and OH⁻ is nearby, it will always convert to a higher coordination, which is consistent with the DFT calculation results on the stability of aluminates in C-A-S-H. The article also provides images of the relaxed bulk structure.
Comparison with Other Studies:
Qomi et al. studied the situation of aluminum substituting for interlayer Ca based on the C-S-H structure of 11Å tobermorite through MD simulations, finding that octahedrally coordinated aluminum forms favorable bonding interactions with silicate chains only if at least one water molecule enters the first coordination shell. However, the results of this study indicate that [AlO₂(OH)₍₄₋ₓ₎(H₂O)ₓ]⁽⁵⁻ˣ⁾ (x = 1, 2) have ICqI values much greater than those observed at 5 ppm in ²⁷Al NMR, and the DFT calculations of this study show that interlayer Al (VI) aluminates are less stable than bridging aluminates, relaxing to five-coordinate geometries with a higher energy penalty. Qomi et al. suggested that their observations align with the ²⁷Al NMR experimental results of Rawal et al. on white Portland cement, who speculated that the broad distribution of ²⁷Al signals around 7 ppm could be attributed to Al (VI) species with water ligands in C-A-S-H (in addition to the usual attribution of the species at 5 ppm to the TAH phase). However, no such signals were observed in the C-A-S-H samples synthesized by the rapid precipitation method in this study. This study believes that another attribution of the 7 ppm signal in the gray oil well cement results by Rawal et al. (i.e., attributed to aluminum-iron phases, whose ²⁷Al NMR signals are broadened due to residual Fe³⁺) is more reasonable, especially considering that Rawal et al. estimated that about 20% of the ²⁷Al NMR signals in their hydrated white Portland cement samples were bleached due to residual Fe³⁺. Qomi et al. also observed that octahedral aluminum can cross-link four Q (1) species, which would inevitably lead to a reduction in interlayer distance (less than 10Å), but the experimental observations contradict this. The interlayer distance of the bulk structure of C-A-S-H after MD relaxation (approximately 1.4ns at 300K) is 13.5Å, while Renaudin et al. reported that the interlayer distance is around 14.5Å when the Ca:Si ratio is less than 1.4, and l’Hopital et al. reported that the interlayer distance of synthesized C-A-S-H samples with a Ca:Si ratio of 1.0 is around 12Å. However, it should be noted that drying samples can significantly affect the measurement of interlayer distances. Currently, there is no other experimental evidence indicating the presence of such cross-linked species in C-(A)-S-H produced from blended cements, and Qomi et al. observed that these parts in molecular modeling may be due to using 11Å tobermorite with cross-linked silicates as the modeling structural framework. Therefore, this study believes that such cross-linked Al (VI) species are unlikely to appear in most C-A-S-H formulations.
Key Notes: / Key notes
Question 1: What factors influence the stability of Al (IV), Al (V), and Al (VI) in C-A-S-H in DFT calculations?
In DFT calculations, the stability of Al (IV), Al (V), and Al (VI) in C-A-S-H is influenced by various factors, including the calcium-silicon ratio (Ca:Si), water-silicon ratio (H₂O:Si), and aluminum-silicon ratio (Al:Si). Specifically, at low Ca:Si ratios, Al (IV) is relatively stable, while Al (VI) has higher energy and is unstable. As the Ca:Si ratio increases, bridging Al (V) and Al (VI) may be more stable than Al (IV). Particularly, at a Ca:Si ratio of 1.75, Al (VI) is approximately 0.3 eV more stable than the corresponding Al (IV) unit. Additionally, increasing the water-silicon ratio also affects the stability of aluminum, as the presence of hydroxyls can promote the stabilization of Al (VI). Calculations also indicate that Al (VI) is more easily stabilized in the presence of more hydroxyls and water molecules.
Question 2: How is the bridging position of Al (VI) in C-A-S-H verified in DNP-enhanced NMR experiments?
In DNP-enhanced NMR experiments, the bridging position of Al (VI) in C-A-S-H is verified by measuring the 2D {29Si}27Al refocused dipolar INEPT correlation spectrum. The specific steps include: first, using DNP technology to enhance the NMR signals of ²⁹Si and ²⁷Al; then, selectively transferring the polarization of ²⁹Si to the nearest neighboring ²⁷Al nuclei through the refocused dipolar INEPT sequence; finally, analyzing the signal distribution in the correlation spectrum. The experimental results show that the Al (VI) signal is strongly correlated with the silicate structure, indicating that this signal originates from the bridging Al (VI) species in C-A-S-H. This is consistent with the results of DFT calculations, further supporting the conclusion that Al (VI) exists in bridging form in C-A-S-H.
Question 3: Traditionally, the 27Al NMR experiment assigns the 5 ppm signal to the “third aluminum hydrate” (TAH) phase, but how does this study’s results revise this conclusion?
This study reassigns the ²⁷Al NMR signals by combining DFT calculations and DNP-enhanced solid-state NMR experiments. Specifically, in C-A-S-H samples with low Al:Si ratios (approximately 0.04), the 1D ²⁷Al CP MAS NMR experiment shows signals for Al (IV), Al (V), and Al (VI), with the Al (VI) signal dominating at 5 ppm. The 2D {²⁹Si}²⁷Al refocused dipolar INEPT correlation spectrum shows that the Al (VI) signal is strongly correlated with the silicate structure, indicating that this signal originates from the bridging Al (VI) species in C-A-S-H. These results contradict the traditional conclusion of assigning the 5 ppm signal to the TAH phase, indicating that the TAH phase does not exist. Instead, the presence of Al (VI) in C-A-S-H is controlled by the interconversion chemistry between aluminum (IV) and Al (VI) driven by water and calcium ions. This finding provides a new perspective for understanding the performance of aluminum-containing cements.
Summary and Supplement: / Feedback
1)Determination of C-A-S-H Atomic Structure: By combining DFT calculation methods based on the C-S-H brick model and DNP-enhanced solid-state NMR experiments, especially the ²⁹Si – ²⁷Al correlation experiments, the researchers have detailed the structure of cementitious (Ca:Si≥1.0) C-A-S-H at the atomic level (as shown in Figure 6 of the paper).
2) Forms and Stability of Aluminates in C-A-S-H:
From an energetic perspective, stable aluminates are incorporated into the silicate chain framework in bridging positions. In the C-A-S-H structure, there are bridging tetrahedral, pentahedral, and octahedral aluminates.
At low Ca:Si ratios, tetrahedral Al (IV) species are more dominant. However, when Ca:Si ≥ 1.2, as long as OH⁻ ions are available as ligands, Al (V) and octahedral Al (VI) species will overall exceed the stability of Al (IV) species. With the increase of additional water and interlayer calcium ions in the system, the availability of OH⁻ also increases, making the bridging [AlO₂(OH)₄]⁵⁻ the most energetically favorable species under high Ca:Si, high H₂O:Si, and low Al:Si ratio conditions.
3) Interconversion of Aluminate Species in C-A-S-H:
Based on the bridging Al (VI) theory, the study discovered a calcium-facilitated, water-driven chemical process for the interconversion between bridging Al (IV) and Al (VI) species. This indicates that calcium and water play direct roles in controlling the aluminum silicate chain structure in C-A-S-H.
4) Attribution of ²⁷Al NMR Signals and Non-Existence of TAH Phase:
Through DNP-enhanced one-dimensional ²⁷Al CP MAS NMR experiments on nearly pure C-A-S-H with low Al:Si ratios (approximately 0.04), signals for Al (IV), Al (V), and Al (VI) were discerned, with the Al (VI) signal at 5 ppm traditionally attributed to the TAH phase dominating in this study.
By calculating the ²⁷Al NMR parameters using DFT, it supports the attribution of the 5 ppm ²⁷Al NMR signal to the [AlO₂(OH)₄]⁵⁻ group. In the case of low Al:Si ratios, these signals still exist during the filtering process of dipolar recoupling from ²⁹Si to ²⁷Al, indicating that they are directly bonded to silicates. Combined with the corresponding two-dimensional ²⁷Al – ²⁹Si DNP enhanced correlation spectra, the study concludes that all these aluminate species, including the signal at 5 ppm, are part of the C-A-S-H structure. This finding aligns with the bridging Al (VI) hypothesis, further confirming the non-existence of the TAH phase.
5) This study conducts extensive and detailed research on the structure of C-A-S-H and the coordination forms of aluminum, providing valuable information for modeling work in computational simulations. Particularly in the supplementary materials, it covers a wealth of microstructural information.
END
Source:Elsevier

Thanks for Reading

