The team led by Florian Hollfelder at the University of Cambridge’s Department of Biochemistry has developed a directed ultra-high-throughput screening platform for biocatalysts in microdroplets, involving NAD(P)H enzymatic reactions and sub-single turnover detection.
Enzyme engineering activities rely on functional screening to discover starting points and subsequent directed evolution. Here, ultra-high-throughput screening forms typically require optical readouts, which may misreport target activity, necessitating custom synthesis. Coupling enzymatic reactions with reporting reactions through common substrates can detect substrates that do not inherently possess fluorescent or colorimetric properties. Improvements in glycoside hydrolases are significant for sustainable biocatalysis.
In this study, the researchers established a detection cascade by linking the release of glucuronic acid residues to the formation of NADH, further connecting it to a novel fluorescent dinitrophenylsulfonyl coumarin probe (SAFRAN) through a specific dehydrogenase acting as a reporter gene, generating fluorescent readouts in response to NAD(P)H through glutathione reductase and subsequent thiol-mediated de-caging reactions. This allows for the quantification of NADH and NADPH-dependent enzyme activity at ultra-high throughput.
To minimize leakage, the researchers incorporated a carboxylic acid group into SAFRAN, applying a negative charge to the coumarin scaffold at physiological pH, enabling its application in droplet microfluidics with minimal leakage. SAFRAN was demonstrated to detect NADH formation and NADPH consumption reactions, exemplifying two representative enzyme classes for droplet-based coupling selection: (i) glycoside hydrolases linked to sugar dehydrogenases as sensor enzymes and (ii) imine reductases consuming NADPH from ketones to synthesize chiral amines.
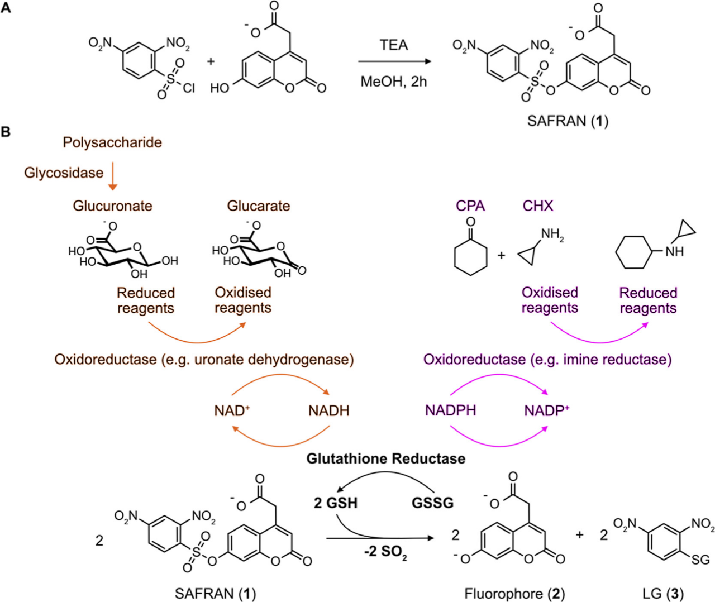
Compared to other NADPH detection methods, the SAFRAN developed in this study exhibits higher sensitivity and reduced inter-droplet leakage, allowing for the detection of low-activity enzymes by extending droplet incubation times. This is crucial for the discovery of protein engineering and other biocatalysts.
Original link:
https://doi.org/10.1021/jacs.4c11804Written by: GPXProofread by: LLEdited by: LL