Life is accompanied by various stresses, and stress is one of the essential characteristics of living organisms. The brain serves as the central hub for vertebrates to perceive and regulate stress, yet there remains a lack of clear understanding regarding how neurons initially sense and transduce stress signals at the cellular level. Research in this area aids in understanding the cellular mechanisms of neuronal stress responses and provides new theories for the prevention and treatment of neuropsychiatric disorders such as anxiety and depression.
Primary cilia are small cellular structures based on microtubules that protrude from the surface of non-dividing cells in vertebrates. They are considered the cell’s antenna, sensing extracellular signals and regulating cellular behavior and function through downstream pathways. Primary cilia structures were discovered in mammalian cortical neurons over 60 years ago, but there is still a lack of understanding regarding their recognized extracellular signals and signaling pathways.
On March 7, 2025, the Shisonghai Research Group from the School of Life Sciences at Tsinghua University, Tsinghua-IDG/McGovern Institute for Brain Research, Beijing Frontier Research Center for Biological Structures, and New Cornerstone Science Laboratory published a research article titled “Primary ciliary protein kinase A activity in the prefrontal cortex modulates stress in mice” in the journal Neuron.This study reveals for the first time a new mechanism by which primary cilia on excitatory neurons in the mouse prefrontal cortex regulate animal stress.

The study observed the morphology of primary cilia in brain neurons and found that two classic animal stress models—chronic restraint stress (CRS) and chronic social defeat stress (CSDS)—both led to a specific increase in primary cilia in the prefrontal cortex (PFC) of stress-related brain regions, suggesting a correlation between primary cilia and animal stress.
To further analyze this correlation, the research team specifically knocked out the key protein CEP83 that regulates primary cilia formation in excitatory neurons of the cortex in mature mice using the Cre/LoxP system, creating a primary cilia-deficient model. The cilia-deficient mice exhibited normal cortical structure, social behavior, and learning and memory functions, but showed a significant decrease in anxiety levels and acute stress response. Furthermore, chronic stress induced depressive-like behavior in wild-type mice, while cilia-deficient mice displayed resistance to chronic stress, exhibiting antidepressant-like characteristics. To further confirm the importance of primary cilia function in regulating stress in mice, the research team specifically knocked out another key protein for primary cilia assembly, TTBK2, creating a second cilia-deficient mouse strain. In these mice, a significant decrease in acute and chronic stress response levels was also observed, further indicating that primary cilia in neurons are involved in the perception and regulation of animal stress.
To elucidate the functional mechanisms of neuronal primary cilia, the research team found that the stress hormone corticosterone similarly caused a significant increase in primary cilia in PFC neurons, and the depressive-like behavior induced by chronic corticosterone injection and the reduced excitability of PFC excitatory neurons depended on the integrity of primary cilia. To determine the core role of the PFC, the research team selectively knocked out primary cilia in excitatory neurons of the PFC using recombinant adeno-associated virus, replicating the changes in stress-related behaviors in mice, while knocking out primary cilia in the sensory cortex did not alter the relevant behaviors in mice.
Finally, the team used a cyclic adenosine monophosphate (cAMP) probe localized to primary cilia and found that corticosterone could cause an increase in cAMP levels within the primary cilia of PFC neurons, while specific inhibition of the downstream protein kinase A (PKA) signaling pathway of cAMP led to a decrease in stress responses and antidepressant-like behavior in mice. These results indicate that primary cilia in excitatory neurons of the mouse PFC perceive the stress hormone corticosterone through the cAMP/PKA signaling pathway, mediating the regulation of neuronal activity related to stress and influencing behavioral phenotypes.
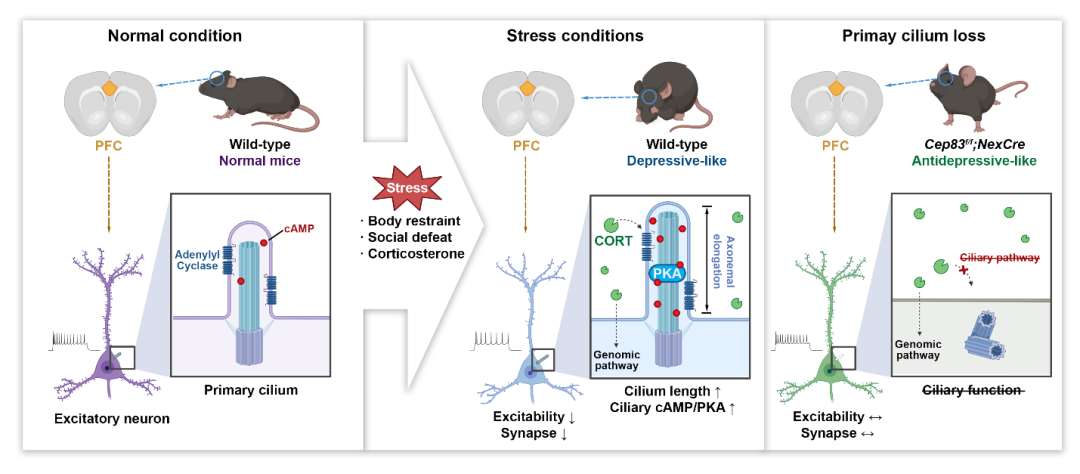 Figure: Protein kinase A signaling regulation of stress in mice by primary cilia in prefrontal cortex neurons
Figure: Protein kinase A signaling regulation of stress in mice by primary cilia in prefrontal cortex neurons
This study fills the gap in understanding the function of primary cilia in neurons within the mature cerebral cortex and opens new directions for understanding the processes of animal stress perception and the formation of depressive-like behaviors.
Dr. Yang Jiajun, a “Shuimu Scholar” at Tsinghua University, is the first author of this article, while Professor Shisonghai from the School of Life Sciences at Tsinghua University is the corresponding author. Researcher Shi Hang from the Center for Structural Biology at Tsinghua University is the co-corresponding author. PhD students Dong Yingjie and Liu Jie from the 2020 cohort at the School of Life Sciences at Tsinghua University made significant contributions to this research, along with PhD students Peng Yuwei (2018 cohort), Wang Ding (2020 cohort), Li Lei (2021 cohort), Hu Xiaoqing (2018 cohort), Li Jinfeng (2024 cohort), and Assistant Researcher Ma Jian who participated in this research work. Researcher Chu Jun and Dr. Wang Liang from the Biomedical Optics Center of the Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences provided valuable cAMP fluorescent probe G-Flamp3 for this study. This research was supported by the major project of Science and Technology Innovation 2030—“Brain Science and Brain-like Research,” the National Natural Science Foundation of China Innovation Group Project, the Beijing Excellent Young Scientist Program, the Beijing Science and Technology Commission Science and Technology Program, and the Beijing Brain Science and Brain-like Research Institute and New Cornerstone Researcher projects.
Paper link:
https://doi.org/10.1016/j.neuron.2025.02.002
Original link:
https://life.tsinghua.edu.cn/info/1131/6571.htm
This article is for academic sharing only; please indicate the source when reprinting. If there is any infringement, please contact WeChat: bioonSir for deletion or modification!