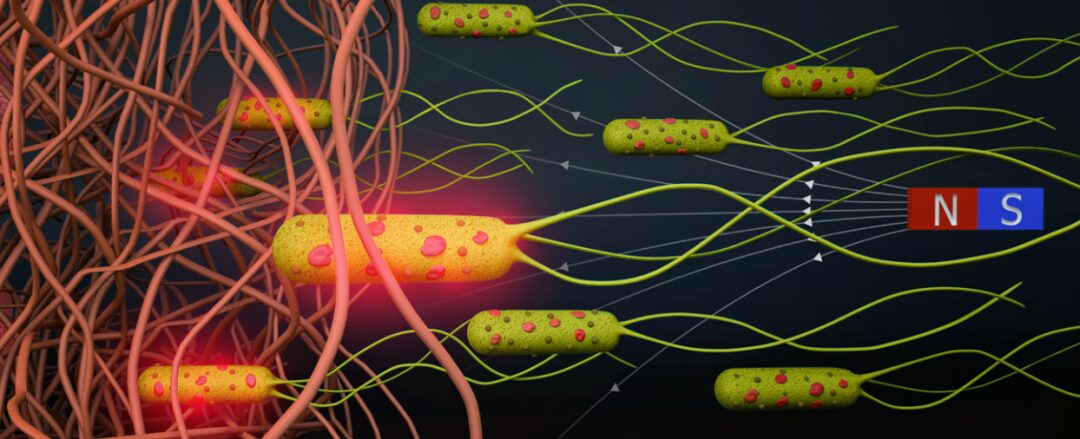

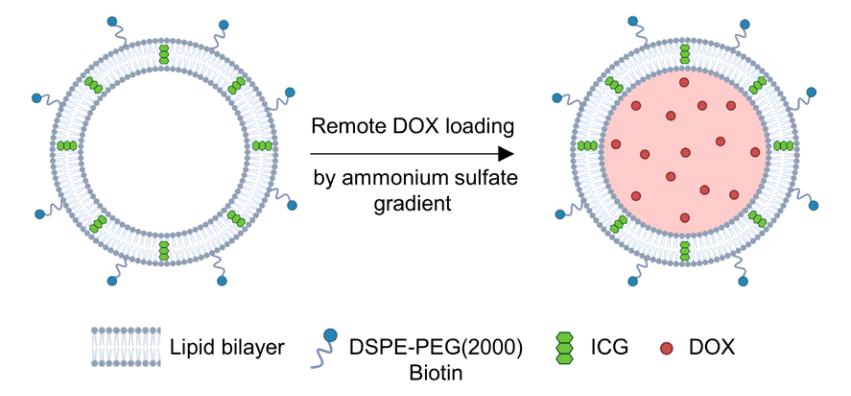
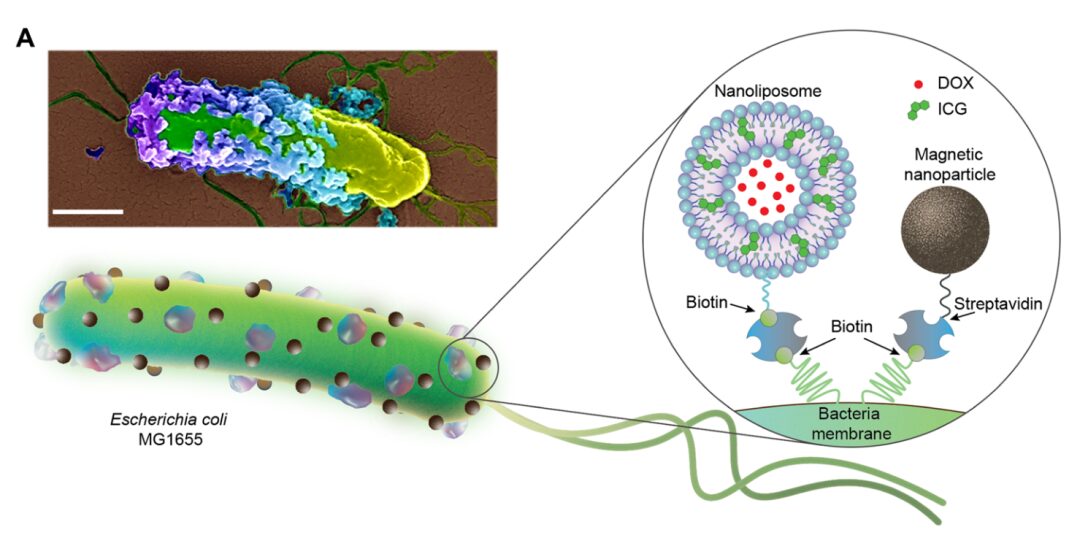
Chinese Society of Biotechnology Science Popularization Account
Follow our updates
Delivering highly valuable popular science knowledge to you









Chinese Society of Biotechnology Science Popularization Account
Follow our updates
Delivering highly valuable popular science knowledge to you

