Click the blue text above to follow us.

Valvular heart disease is a significant aspect of structural heart disease, withmitral regurgitation being the most common among them. Surveys indicate that the prevalence of mild, moderate, and severe mitral regurgitation in the United States is 19.2%, 1.6%, 0.3%, and 0.2%, respectively. It is estimated that over 250,000 patients are diagnosed with mitral regurgitation annually in the U.S., with similar prevalence rates in Europe.
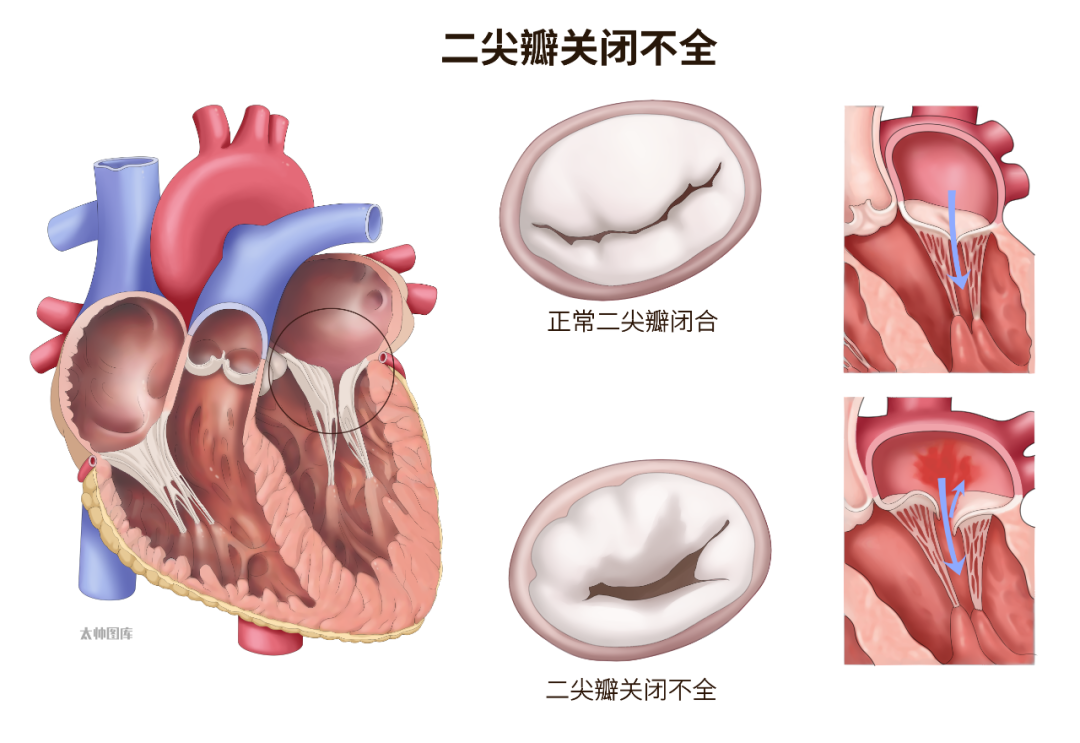
Although the prevalence of mitral regurgitation in China is not clearly defined, conservative estimates suggest that the total number of patients has already exceeded ten million.The normal structure of the mitral valve consists of the annulus, leaflets, chordae tendineae, papillary muscles, and the associated left ventricular wall; defects in any of these components can lead to mitral regurgitation.Mitral regurgitation can be classified based on its pathogenesis intoprimary mitral regurgitation and secondary mitral regurgitation. Primary mitral regurgitation is caused by structural abnormalities of the mitral valve, also known as organic mitral regurgitation; secondary mitral regurgitation results from annular dilation, left atrial and/or left ventricular enlargement, papillary muscle displacement, and left ventricular dysfunction, thus referred to as functional mitral regurgitation.Recent studies abroad have reported that patients with atrial fibrillation (AF) can develop secondary functional mitral regurgitation, and this type of functional mitral regurgitation associated with AF-induced left atrial remodeling is termed atrial functional mitral regurgitation (AFMR).Previous studies in Western countries have reported an incidence of AFMR ranging from 2.8% to 66.7%, while there is currently no large-scale data on the incidence of AFMR in China. Given the high prevalence of AF globally and the large patient population, along with the accelerating aging process, it is expected that the incidence of AFMR may continue to rise, and thus this specific type of functional mitral regurgitation should not be overlooked.
The Pathogenesis of AFMR
01
Isolated Annular Dilation
Persistent atrial fibrillation leads to left atrial enlargement, which subsequently causes dilation of the mitral valve annulus, resulting in functional mitral regurgitation.Kihara et al. found that patients with mitral regurgitation and atrial fibrillation had significantly enlarged mitral valve annuli and left atria, suggesting that atrial fibrillation-induced left atrial enlargement is a primary cause of mitral regurgitation in these patients.Kim et al. used three-dimensional echocardiographic Doppler technology to conduct detailed examinations and analyses of patients with mitral regurgitation and atrial fibrillation, discovering that atrial fibrillation can lead to left atrial enlargement, causing mitral valve annulus dilation; they also found that the dilation of the mitral valve annulus was asymmetric, with the anterior leaflet expanding less than the posterior leaflet, and the maximum stretch point shifting posteriorly. These findings further validate the hypothesis that isolated annular dilation leads to AFMR.
02
Atrial Leaflet Tethering
Compared to the posterior leaflet of the mitral valve, which is attached to the junction of the left atrium and left ventricular free wall, the anterior leaflet, which is attached to the aortic root, is more fixed and less prone to displacement. Persistent atrial fibrillation can lead to left atrial enlargement, mitral valve annulus dilation, and outward expansion of the left ventricular free wall, causing the posterior leaflet to shift, resulting in systolic bulging of the posterior leaflet away from the annular plane, leading to poor coaptation of the mitral leaflets, resulting in atrial functional mitral regurgitation. Additionally, left atrial enlargement can cause the mitral valve annulus plane to shift upward, increasing the distance between the annulus and the papillary muscles, exacerbating mitral regurgitation.
03
Posterior Leaflet Motion Impairment
When the left atrium enlarges, the base of the left ventricular posterior wall is pulled towards the left ventricular cavity. During systole, as the left ventricular pressure increases, the base of the left ventricular posterior wall moves outward, at which point, the posterior leaflet of the mitral valve experiences opposing forces from two directions, leading to motion impairment (one force is the tethering force from the papillary muscles towards the left ventricular cavity, and the other is the outward force from the left ventricular cavity), which can subsequently lead to leaflet closure failure and regurgitation.
04
Impaired Annular Contraction and Flattening of the Saddle Shape
The mitral valve annulus is a passively swinging fibrofatty ring, and its movement depends on the motion of the aortic root and the contraction and relaxation of the adjacent left atrial and left ventricular muscle groups. Under normal physiological conditions, the area of the annulus can decrease by 25% due to effective contraction, further promoting leaflet closure. In patients with persistent atrial fibrillation, the effective contraction of the annulus is reduced, leading to an increased area required for closure, while the flattening of the saddle shape of the mitral valve annulus can further increase leaflet stress and tethering distance, subsequently leading to mitral regurgitation.
05
Insufficient or Excessive Compensatory Proliferation of the Mitral Leaflets
When atrial fibrillation causes left atrial remodeling leading to mitral regurgitation, compensatory proliferation of the mitral leaflets may occur to reduce mitral regurgitation and increase effective closure; however, the proliferation of the leaflets is often insufficient, resulting in atrial functional mitral regurgitation. However, excessive compensatory proliferation can also lead to impaired leaflet motion, affecting effective closure and exacerbating regurgitation.
06
Left Atrial and Left Ventricular Dysfunction
Persistent atrial fibrillation is often a chronic condition, and during the prolonged disease progression, the left atrium gradually undergoes fibrosis, severely affecting its function, and this process also affects the compliance of the left ventricle, thereby impacting normal atrioventricular activity, which can induce mitral regurgitation.
Pathophysiology of AFMR
Under physiological conditions, the anterior leaflet of the mitral valve is connected to the aortic root and is relatively fixed; the posterior leaflet is supported by the muscle at the junction of the left atrium and left ventricle, and its endocardium is connected to the endocardium of the posterior wall of the left atrium, making its position susceptible to change. Therefore, when the left atrium enlarges, it can lead to tethering of the posterior leaflet, subsequently limiting its closure, thus affecting the effective closure of the posterior leaflet and causing functional mitral regurgitation. It is important to note that left atrial enlargement is closely related to atrial fibrillation. Atrial fibrillation can induce left atrial enlargement, leading to limited tethering of the posterior leaflet and resulting in functional mitral regurgitation. Furthermore, mitral regurgitation can further exacerbate left atrial and left ventricular enlargement, increasing the degree of mitral valve annulus dilation and leaflet tethering, thereby worsening mitral regurgitation. Thus, this functional mitral regurgitation can be mutually causal with atrial fibrillation, interacting and progressively worsening.
Transcatheter Minimally Invasive Treatment of AFMR
Catheter Ablation
Catheter ablation refers to the placement of multiple multi-polar electrode catheters within the heart through venous or arterial access, using cardiac electrophysiological techniques to map and locate within the heart, then positioning the ablation catheter electrodes at the sites of arrhythmia or abnormal conduction pathways, applying high-energy current, laser, radiofrequency current, cytotoxic substances, cryoablation, and ultrasound methods to induce necrosis or damage to the myocardium in that area, achieving the goal of treating refractory arrhythmias.Since the discovery of ectopic foci in the pulmonary veins leading to atrial fibrillation in 1998, the ablation treatment for atrial fibrillation has rapidly developed. From the initial focal ablation of the pulmonary veins, it has evolved into segmental pulmonary vein isolation and currently includes various methods such as linear ablation of the left atrium. Due to the high recurrence rate of focal ablation and the tendency for segmental pulmonary vein isolation to cause pulmonary vein stenosis, linear ablation of the left atrium is now commonly used, isolating both pulmonary veins at the junction with the left atrium and adding complex fragmented potential ablation and linear ablation of the left atrial isthmus and roof based on the patient’s specific condition, and if necessary, adding linear ablation of the right atrium and isolation of the superior vena cava.Currently, the success rate of catheter ablation for atrial fibrillation can reach 90% both domestically and internationally, making it a first-line choice for treating atrial fibrillation.As understanding of AFMR deepens, more studies have shown that catheter ablation to restore sinus rhythm helps improve atrial functional mitral regurgitation.Gertz et al. conducted a retrospective study on 53 patients with atrial fibrillation and moderate or greater mitral regurgitation, examining the left atrium, mitral valve annulus, and overall mitral regurgitation status before and after ablation to restore sinus rhythm, finding significant reductions in both the left atrium and annulus, with marked improvement in mitral regurgitation, indicating that catheter ablation to restore sinus rhythm aids in improving atrial functional mitral regurgitation.
Nishino et al. led a prospective study involving 119 patients with atrial fibrillation undergoing catheter ablation, performing transthoracic and transesophageal echocardiography before and after the procedure. Six months post-operation, they found significant improvements in mitral valve annulus contraction, orifice area, and regurgitation severity in patients who restored sinus rhythm after catheter ablation compared to pre-operation. This study also first demonstrated the reverse remodeling effect of restoring sinus rhythm on the mitral valve post-ablation, further proving the improvement of atrial functional mitral regurgitation after restoring sinus rhythm post-ablation.Catheter ablation is simple, effective, and minimally invasive, effectively alleviating regurgitation in patients with AFMR; however, for patients with severe AFMR and significantly enlarged left atria, the recurrence rate of atrial fibrillation post-ablation is high, and the reversal of left atrial and mitral valve function is not significant, thus limiting its ability to improve late-stage atrial functional mitral regurgitation. Therefore, for patients with AFMR, catheter ablation should be applied early to timely intervene in atrial fibrillation, aiming to reverse cardiac structure in the early to mid-stages of the disease and eliminate functional mitral regurgitation to prevent further deterioration and progression of the disease.
Transcatheter Edge-to-Edge Repair
The edge-to-edge repair technique in surgical mitral valve repair is simple and unique, first pioneered by Italian surgeon Otavio Alfieri in the 1990s.This surgery sutures the middle of the anterior leaflet to the middle of the posterior leaflet, transforming the mitral orifice from a large single orifice during systole into a small double orifice, thereby reducing the volume of mitral regurgitation; hence, this technique is also known as the “double-orifice technique.” Compared to traditional leaflet repair techniques, this method does not require consideration of the causes, damage, and anatomical conditions of mitral regurgitation, and although it is simple to perform, it has shown satisfactory clinical efficacy, proven safe and effective in patients with degenerative and functional lesions.Inspired by surgical edge-to-edge mitral valve repair, physicians have developed transcatheter edge-to-edge repair (TEER). This procedure employs principles similar to those of surgical edge-to-edge mitral valve repair, performed under general anesthesia, using a specially designed mitral valve clip, entering through the femoral vein, puncturing the interatrial septum to access the left atrium and left ventricle, and under the guidance of three-dimensional ultrasound and digital subtraction angiography, the clipper grasps the middle of the anterior and posterior leaflets, transforming the mitral orifice from a large single orifice into a small double orifice, thereby reducing mitral regurgitation.TEER has significant advantages over surgical procedures: surgical operations are invasive, have long recovery times, require support from cardiopulmonary bypass, and many elderly and high-risk patients cannot tolerate open-heart surgery, posing significant surgical risks; TEER does not require thoracotomy or cardiopulmonary bypass support, allowing instruments to be delivered to the heart via the femoral vein, with a short operation time and minimally invasive approach, leading to rapid recovery for patients, making it a boon for elderly, high-risk patients with contraindications for surgical mitral regurgitation. Yoshida et al. conducted a retrospective study on 155 patients who underwent transcatheter edge-to-edge repair (40 AFMR patients and 115 patients with functional mitral regurgitation in sinus rhythm), analyzing transesophageal echocardiographic data, finding significant reductions in annulus area, regurgitation, and anterior-posterior diameter in both groups post-operation, with similar rates of residual regurgitation at 12 months post-operation. These results suggest that while the clinical efficacy of edge-to-edge repair for AFMR patients is slightly inferior to that for patients with functional mitral regurgitation in sinus rhythm, the overall level is still acceptable, demonstrating the effectiveness of edge-to-edge repair for AFMR patients.
In 2021, the application of edge-to-edge repair in cases of severe AFMR with significant mitral valve annulus calcification was first reported in the JACC journal, with the patient experiencing a reduction in mitral regurgitation to mild immediately post-operation, showing significant surgical effects.
Transcatheter Mitral Valve Replacement
As TEER continues to develop, transcatheter mitral valve replacement is also undergoing gradual exploration and development. Unlike transcatheter aortic valve replacement via peripheral access, transcatheter mitral valve replacement mostly employs a transapical approach, likely due to the larger size of the artificial interventional valve and the longer peripheral venous access (which is harder to manipulate), while the transapical approach effectively avoids these issues with a more direct and shorter path.Transcatheter mitral valve replacement must be performed under general anesthesia with transesophageal echocardiography and fluoroscopic guidance, making a 4 cm incision at the apex of the heart, placing the transcatheter mitral valve replacement delivery system, successfully crossing the valve, and performing angiography to assess the position of the artificial valve. After adjusting the position and depth, the left ventricular anchoring device is gradually released under ultrasound guidance, capturing the leaflets, and once the leaflets are captured, the valve position is readjusted, and the valve is released. After the release is completed, angiography and ultrasound evaluation of the release results are performed, and the apex incision is sutured to complete the operation.Currently, transcatheter mitral valve replacement is mainly applied in secondary surgeries for damaged artificial bioprosthetic valves/artificial annuloplasty rings and in cases of severe calcification of the mitral valve annulus, while its application in truly non-calcified mitral regurgitation patients is limited, and even less so in AFMR cases.Although there is still a significant distance to the widespread application of transcatheter mitral valve replacement for AFMR, it remains a potential treatment option for AFMR, warranting further research and exploration.
AFMR is an important aspect of functional mitral regurgitation that cannot be overlooked; this disease has a high incidence and is often associated with other conditions, and if not addressed promptly, the clinical prognosis is extremely poor. Therefore, early intervention and timely management of this disease are necessary.
Transcatheter minimally invasive treatment can effectively alleviate atrial functional mitral regurgitation under the premise of simplicity and minimal invasiveness, delay disease progression, and improve preoperative discomfort symptoms, making it an important method for treating elderly, high-risk patients with contraindications for surgical intervention in AFMR.
END
Source: Zhang Hang, Shi Fengwu. Transcatheter Minimally Invasive Treatment of Atrial Functional Mitral Regurgitation. Chinese Journal of Interventional Cardiology, 2022, 30(09)
References
1 Wang Bin, Chen Xiang, Su Maolong, et al. First Report of a New Generation of Domestic Transcatheter Edge-to-Edge Repair System via Femoral Vein for High-Risk Functional Mitral Regurgitation. Chinese Journal of Interventional Cardiology, 2021, 29(7): 416-418.
2 Jones EC, Devereux RB, Roman MJ, et al. Prevalence and correlates of mitral regurgitation in a population-based sample (the Strong Heart Study). Am J Cardiol, 2001, 87(3): 298-304.
3 Ge Junbo, Zhou Daxin, Pan Wenzhi. Transcatheter Heart Valve Therapy. Shanghai: Shanghai Science and Technology Press, 2013: 89-97.
4 Nkomo VT, Gardin JM, Skelton TN, et al. Burden of valvular heart diseases: a population-based study. Lancet, 2006, 368(9540): 1005-1011.
5 Pan Wenzhi, Zhou Daxin, Ge Junbo. New Understanding of the Mechanism of Mitral Regurgitation and Its Application. Chinese Journal of Medical Frontier (Electronic Edition), 2017, 9(10): 43-46.
6 Abe Y, Takahashi Y, Shibata T. Functional mitral regurgitation, updated: ventricular or atrial. J Echocardiogr, 2020, 18(1): 1-8.
7 Machino-Ohtsuka T, Seo Y, Ishizu T, et al. Novel mechanistic insights into atrial functional mitral regurgitation: 3-dimensional echocardiographic study. Circ J, 2016, 80(10): 2240-2248.
8 Kagiyama N, Hayashida A, Toki M, et al. Insufficient leaflet remodeling in patients with atrial fibrillation: association with the severity of mitral regurgitation. Circ Cardiovasc Imaging, 2017, 10(3): e005451.
9 Itabashi Y, Mihara H, Berdejo J, et al. Distant position of chordae from coaptation causes mitral regurgitation in patients with atrial fibrillation. J Heart Valve Dis, 2016, 25.
10 Wang Bin, Chen Xiang, Su Maolong, et al. First Report of a New Generation of Domestic Transcatheter Edge-to-Edge Repair System via Femoral Vein for High-Risk Functional Mitral Regurgitation. Chinese Journal of Interventional Cardiology, 2021, 29(7): 416-418.
11 Jones EC, Devereux RB, Roman MJ, et al. Prevalence and correlates of mitral regurgitation in a population-based sample (the Strong Heart Study). Am J Cardiol, 2001, 87(3): 298-304.
12 Ge Junbo, Zhou Daxin, Pan Wenzhi. Transcatheter Heart Valve Therapy. Shanghai: Shanghai Science and Technology Press, 2013: 89-97.
13 Nkomo VT, Gardin JM, Skelton TN, et al. Burden of valvular heart diseases: a population-based study. Lancet, 2006, 368(9540): 1005-1011.
14 Pan Wenzhi, Zhou Daxin, Ge Junbo. New Understanding of the Mechanism of Mitral Regurgitation and Its Application. Chinese Journal of Medical Frontier (Electronic Edition), 2017, 9(10): 43-46.
15 Abe Y, Takahashi Y, Shibata T. Functional mitral regurgitation, updated: ventricular or atrial. J Echocardiogr, 2020, 18(1): 1-8.
16 Machino-Ohtsuka T, Seo Y, Ishizu T, et al. Novel mechanistic insights into atrial functional mitral regurgitation: 3-dimensional echocardiographic study. Circ J, 2016, 80(10): 2240-2248.
17 Kagiyama N, Hayashida A, Toki M, et al. Insufficient leaflet remodeling in patients with atrial fibrillation: association with the severity of mitral regurgitation. Circ Cardiovasc Imaging, 2017, 10(3): e005451.
18 Itabashi Y, Mihara H, Berdejo J, et al. Distant position of chordae from coaptation causes mitral regurgitation in patients with atrial fibrillation. J Heart Valve Dis, 2016, 25.