Click the blue text
Follow us
This
Issue

Highlights
Currently, the only approved TF-ADC drug globally is Seagen’s TIVDAK (tisotumab vedotin-tftv, for cervical cancer), with a carrier of VA–MMAE. It has been approved for sale in Japan. The domestic sales are handled by Zai Ding Pharmaceutical, which has submitted a listing application. Xin Nuo Wei’s TF-ADC (XNW28012) has chosen pancreatic cancer as a differentiated indication, demonstrating significant strategic innovation and sincerely hopes to achieve breakthroughs in the “king of cancers” pancreatic cancer field.
This issue includes
|
01 |
Xin Nuo Wei | TF ADC | Pancreatic Cancer |
|
02 |
Seagen | Zai Ding | TF ADC | Cervical Cancer |
|
03 |
ADC—A New Era of Precision Therapy |
|
04 |
Summary and Outlook |
【01 Xin Nuo Wei | TF ADC | Pancreatic Cancer】
On June 30, 2025, Xin Nuo Wei Pharmaceutical registered a Phase III randomized, double-blind, multicenter study (CTR20252545) targeting patients with metastatic pancreatic cancer who have previously received systemic therapy.
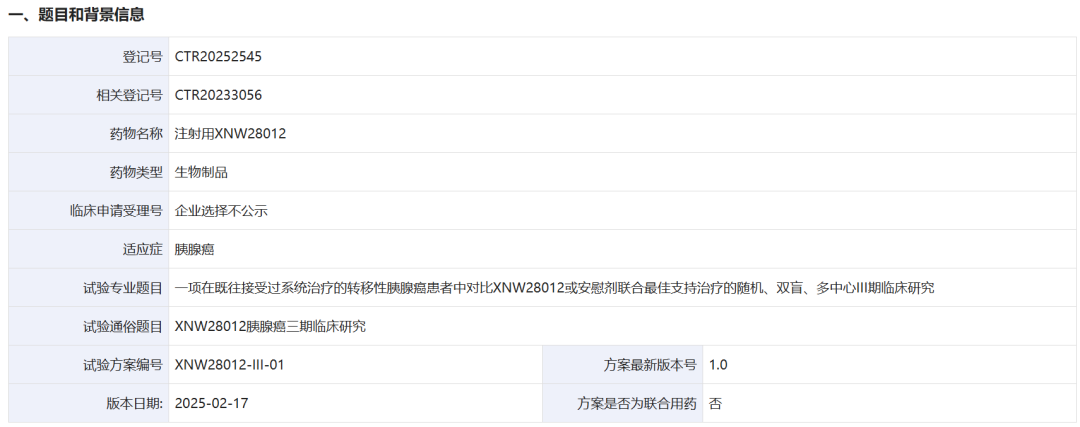
This study will compare the efficacy and safety of the new drug XNW28012 combined with the best supportive care versus placebo combined with the best supportive care, planning to enroll 150 cases, with overall survival (OS) as the primary endpoint. Dr. Yu Xianjun from Fudan University Shanghai Cancer Center serves as the principal investigator. This is the first Phase III clinical trial globally targeting the tissue factor (TF) target in pancreatic cancer.
XNW28012
XNW28012 is designed based on the Yilian Biotech TMALIN platform, using a tumor microenvironment activable linker to conjugate a novel isomerase inhibitor toxin YL0014, with a drug-to-antibody ratio (DAR) of up to 8.
This is the second ADC under development by Xin Nuo Wei. The first one is CLDN18.2 ADC.
On May 30, 2025, Xin Nuo Wei Pharmaceutical partnered with AstraZeneca to reach an exclusive licensing agreement for the clinical-stage next-generation targeted CLDN18.2 antibody-drug conjugate XNW27011. Under the agreement, Xin Nuo Wei grants AstraZeneca exclusive development and commercialization rights for XNW27011 globally (excluding mainland China, Hong Kong, Macau, and Taiwan).

According to the terms of the agreement, Xin Nuo Wei can receive an initial payment of $130 million, with the opportunity to obtain up to $70 million in near-term payments, and up to a total of $1.34 billion in milestone payments covering development, registration, and commercialization. Additionally, from the time XNW27011 is approved for sale, Xin Nuo Wei will receive corresponding royalties based on the net sales of the product.
For details, see:
AstraZeneca Spends $1.54 Billion to Acquire CLDN18.2 ADC: Is it a Failure of Monoclonal Antibodies or a Strategic Vision?

02 Seagen | Zai Ding | TF ADC | Cervical Cancer
TF Target
Tisotumab vedotin is a novel ADC drug that includes a monoclonal antibody targeting tissue factor (TF) and a microtubule-disrupting agent—monomethyl auristatin E (MMAE). TF is abnormally expressed in various solid tumors, promoting tumor growth, neovascularization, and accelerating tumor metastasis. After tisotumab vedotin binds to and internalizes with tumor cell surface TF, it releases MMAE to induce cytotoxicity, effectively killing tumor cells and demonstrating excellent therapeutic effects in cervical cancer.
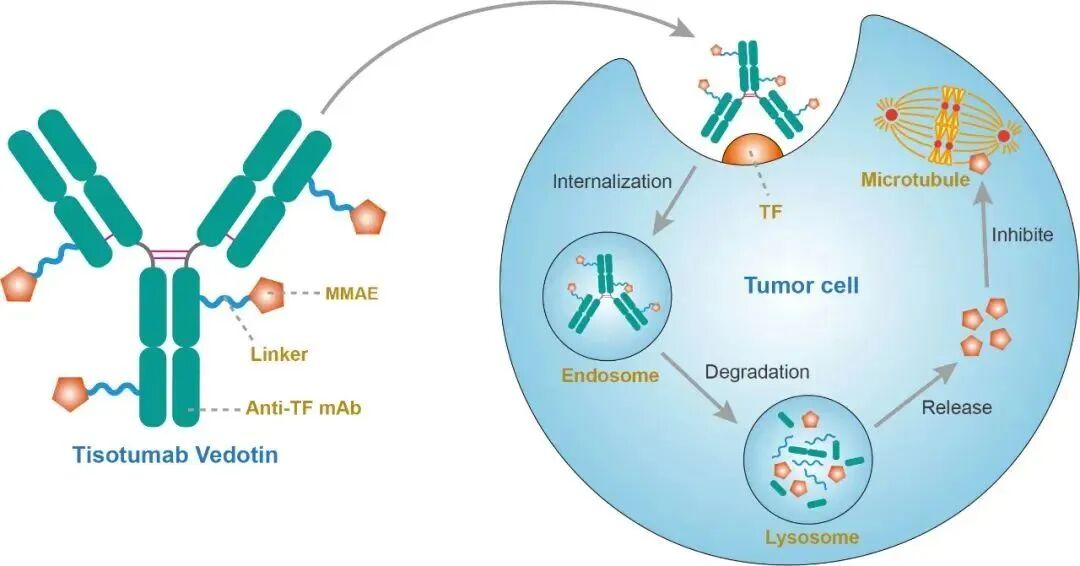
Mechanism of action of tisotumab vedotin
In early 2024, Genmab and Seagen jointly announced that their innovaTV 301 global Phase III trial achieved its primary endpoint of overall survival (OS). Thus, Tivdak is the first ADC approved for the treatment of recurrent or metastatic cervical cancer patients with disease progression during or after chemotherapy. In April 2024, the FDA fully approved the use of tisotumab vedotin for the treatment of recurrent or metastatic cervical cancer patients with disease progression during or after chemotherapy.
Zai Ding Pharmaceutical | Introduction of TF ADC
Zai Ding Pharmaceutical agreed to collaborate with Seagen in 2022 to develop and commercialize TIVDAK (tisotumab vedotin). According to the agreement, the company obtained exclusive rights to develop and commercialize TIVDAK in mainland China, Hong Kong, Macau, and Taiwan from Seagen.
According to the terms of the agreement, the company will pay Seagen a $30 million upfront payment, plus up to a total of $78 million in development and registration milestone payments upon reaching specified development and registration milestones, and up to a total of $185 million in sales milestone payments upon reaching specified sales milestones. Seagen will also be entitled to receive certain royalties based on the annual net sales of the licensed product within the licensed territory, with reductions possible under specific circumstances.

Source: Zai Ding Pharmaceutical Official Website
TIVDAK is currently advancing in Phase II clinical research for first-line cervical cancer, hoping to achieve good efficacy results in first-line cervical cancer as well.
【03 ADC—A New Era of Precision Therapy】
ADC therapy delivers cytotoxic agents precisely through a “biological missile” model, becoming a disruptive force in the treatment of solid tumors and hematological malignancies.
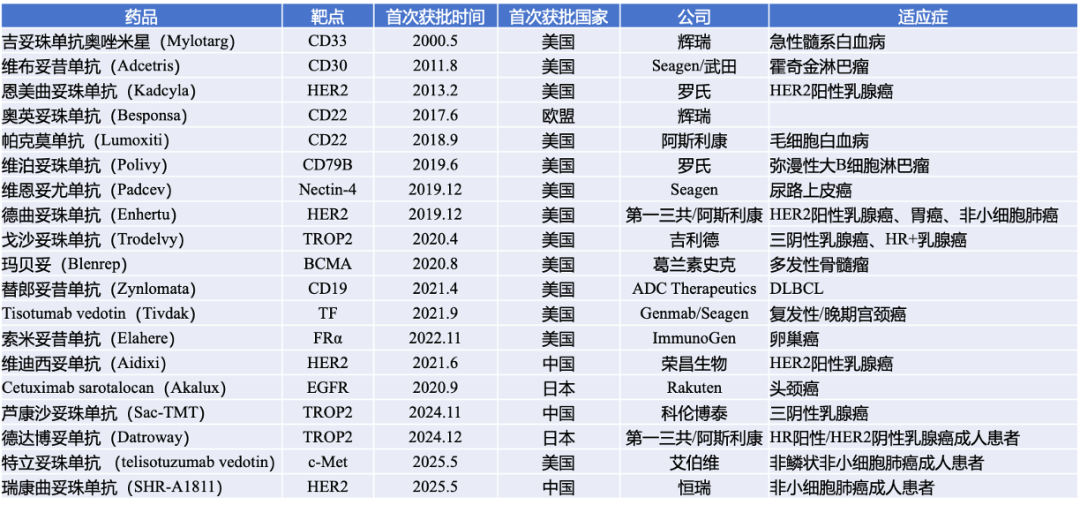
As of May 2025, the global ADC landscape has exploded, with 19 drugs covering targets such as HER2, Trop-2, and CLDN18.2, with continuous breakthroughs in technology iteration and application boundaries. In the competition for the potential TF target, Xin Nuo Wei’s differentiated choice of pancreatic cancer, if successful in Phase III, will mean the arrival of the era of precision therapy in the pancreatic cancer field.
04 Summary and Outlook
For this truly focused approach to clinically difficult-to-treat tumors, with both drug targets and drug forms being genuinely innovative, we give a thumbs up. We hope the Chinese biotech industry actively seizes the recent bull market, accelerates the expansion of clinical layouts, and benefits a broader range of patients, which is the original intention of drug developers.
★
